| Identification | Back Directory | [Name]
3-OXO-4-PHENYL-BUTYRIC ACID ETHYL ESTER | [CAS]
718-08-1 | [Synonyms]
Ethyl 4-phenylacet
Ethyl 4-phenylacetoacetate
Ethyl 3-Oxo-4-phenylbutyrate
ethyl 3-oxo-4-phenylbutanoate
ethyl 3-oxo-4-phenyl-butanoate
b-Oxo-benzenebutanoic acid ethyl ester
3-OXO-4-PHENYL-BUTYRIC ACID ETHYL ESTER
4-Phenyl-3-oxobutanoic acid ethyl ester
3-keto-4-phenyl-butyric acid ethyl ester
Benzenebutanoic acid, β-oxo-, ethyl ester
Benzenebutanoic acid, b-oxo-, ethyl ester
? Benzenebutanoic acid, β-oxo-, ethyl ester | [EINECS(EC#)]
226-500-0 | [Molecular Formula]
C12H14O3 | [MDL Number]
MFCD03844818 | [MOL File]
718-08-1.mol | [Molecular Weight]
206.24 |
| Chemical Properties | Back Directory | [Boiling point ]
290℃ | [density ]
1.091 | [refractive index ]
1.054-1.059 | [Fp ]
124℃ | [storage temp. ]
Inert atmosphere,Room Temperature | [solubility ]
Chloroform (Slightly), DMSO (Slightly), Methanol (Sparingly) | [form ]
Oil | [pka]
10.49±0.46(Predicted) | [color ]
Colourless to Light Yellow |
| Hazard Information | Back Directory | [Chemical Properties]
Light-yellow Liquid. | [Uses]
Ethyl 4-Phenylacetoacetate is a useful synthetic intermediate. It is used to prepare pyrazolone derivatives as antiprion compounds. It is also used to prepare pyrrolinylaminopyrimidine analogs as inhibitors of AP-1 and NF-κB mediated gene expression. | [Synthesis Reference(s)]
Journal of Medicinal Chemistry, 44, p. 78, 2001 DOI: 10.1021/jm001034k
Chemical and Pharmaceutical Bulletin, 30, p. 2440, 1982 DOI: 10.1248/cpb.30.2440 | [Synthesis]
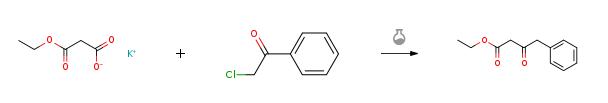
The synthesis of Ethyl 3-oxo-4-phenylbutanoate is as follows:Monoethyl monopotassium malonate (12.9 g, 2.3 equivalents) was mixed with tetrahydrofuran (200 ml), and the mixture was cooled to 5°C. Triethylamine (8.2 g, 2.5 equivalents) and magnesium chloride (8.62 g, 2.8 equivalents) were added, and the mixture was stirred at 5 to 20°C for 3 hours. The reaction mixture was cooled to 5°C. Phenacyl chloride (5 g, 32 mmol, 1 equivalent) was gradually added, and the mixture was stirred at 5 to 20°C for 63 hours. The mixture was cooled to 5°C, and 1 N hydrochloric acid (30 ml) was added. Tetrahydrofuran was evaporated away under reduced pressure, and extraction was carried out with ethyl acetate (50 ml). The organic layer was washed with 1 N hydrochloric acid (30 ml), water (10 ml), saturated aqueous solution of sodium hydrogencarbonate (30 ml) and water (10 ml) in order. The solvent was evaporated away under reduced pressure to obtain the Ethyl 3-oxo-4-phenylbutanoate as a pale yellow oil (5.82 g, yield: 86 %).
 |
|
|