| Identification | Back Directory | [Name]
TERT-BUTYL 4-BROMOBENZYLCARBAMATE | [CAS]
68819-84-1 | [Synonyms]
N-BOC-4-broMobenzylaMine
4-Bromo-N-BOC-benzylamine
TERT-BUTYL 4-BROMOBENZYLCARBAMATE
tert-Butyl 4-bromobenzylcarbamate 98%
tert-Butyl N-(4-bromobenzyl)carbamate
tert-butyl N-[(4-bromophenyl)methyl]carbamate
(4-BROMO-BENZYL)-CARBAMIC ACID TERT-BUTYL ESTER
N-(4-Bromobenzyl)carbamic acid tert-butyl ester
Carbamic acid, (4-bromophenyl)methyl-, 1,1-dimethylethyl ester
Carbamic acid, N-[(4-bromophenyl)methyl]-, 1,1-dimethylethyl ester | [Molecular Formula]
C12H16BrNO2 | [MDL Number]
MFCD08703140 | [MOL File]
68819-84-1.mol | [Molecular Weight]
286.16 |
| Chemical Properties | Back Directory | [Melting point ]
86-88℃ | [Boiling point ]
374.3±25.0 °C(Predicted) | [density ]
1.319±0.06 g/cm3(Predicted) | [storage temp. ]
Sealed in dry,Room Temperature | [form ]
solid | [pka]
12.05±0.46(Predicted) | [color ]
Off-white |
| Hazard Information | Back Directory | [Synthesis]
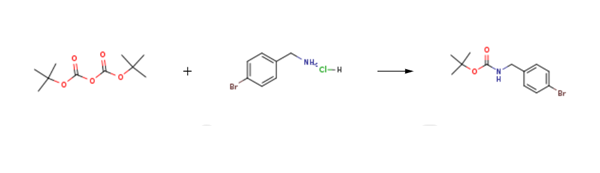
Tert-Butyl 4-bromobenzylcarbamate is prepared by the reaction of di-tert-butyl dicarbonate and 4-bromobenzylamine hydrochloride. The specific synthesis steps are as follows:
To a stirred suspension of 4-bromo-benzylamine hydrochloride (968mg, 4.35mmol) and triethylamine (0.666ml, 4.79mmol) in chloroform (15ml) was added di-tert-butyldicarbonate (949mg, 4.35mmol). The resulting solution was stirred at ambient temperature for 2h and then diluted with DCM (40ml). The organic solution was washed sequentially with 10% aqueous citric acid (40ml) and brine (40ml). The organic phase was dried over anhydrous magnesium sulfate and the filtrate evaporated at reduced pressure to afford Tert-Butyl 4-bromobenzylcarbamate (1.23g, 99%). 1H NMR 7.48-7.43 (2H, m), 7.18 (2H, d, 8.4), 4.84 (1H, br s), 4.27 (2H, d, 5.7), 1.46 (9H, s).
 |
|
|