| Identification | Back Directory | [Name]
ETHYL 4,6-DICHLOROPYRIDAZINE-3-CARBOXYLATE | [CAS]
679406-03-2 | [Synonyms]
EOS-61510
4,6-DICHLOROPYRIDAZINE-3-CARBOXYLATE
ETHYL 4,6-DICHLOROPYRIDAZINE-3-CARBOXYLATE
4,6-Dichloro-pyridazine-3-carboxylic acid ethyl ester
3-Pyridazinecarboxylic acid, 4,6-dichloro-, ethyl ester | [Molecular Formula]
C7H6Cl2N2O2 | [MDL Number]
MFCD11521570 | [MOL File]
679406-03-2.mol | [Molecular Weight]
221.04 |
| Chemical Properties | Back Directory | [Boiling point ]
353.4±37.0 °C(Predicted) | [density ]
1.433±0.06 g/cm3(Predicted) | [storage temp. ]
Inert atmosphere,Store in freezer, under -20°C | [solubility ]
Acetonitrile (Slightly), Chloroform (Soluble), Methanol (Slightly) | [form ]
Low-Melting Solid | [pka]
-2.18±0.10(Predicted) | [color ]
Brown to Very Dark Brown |
| Hazard Information | Back Directory | [Uses]
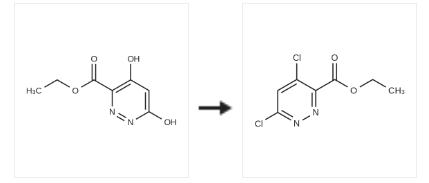
Ethyl 4,6-Dichloropyridazine-3-carboxylate is a useful reagent for the green preparation of Deucravacitinib, a deuterated API for TYK2 Inhibition. | [Synthesis]
To a 350 mL nitrogen purged Schlenk flask containing Ethyl 4,6-dihydroxypyridazine-3-carboxylate (3.77 g, 20.47 mmol) was added phosphorus oxychloride (38 mL, 408 mmol). The vessel was sealed and heated to 100 °C for 3.5 hours. The reaction was cooled to room temperature and the excess phosphorus oxychloride was removed in vacuo. The crude oil was dissolved into chloroform, re-concentrated and then poured into ice water, rinsing with ethyl acetate.The two layers were transferred to a separatory funnel, separated and the aqueous layer extracted 3x with ethyl acetate. The combined organic layers were washed twice with water and once with brine (saturated aqueous sodium chloride) and then dried over sodium sulfate, filtered, concentrated and then purified by automated chromatography (5- 90percent EtOAc:hexanes), providing Ethyl 4,6-dichloropyrridazine-3-carboxylate (3.64 g, 16.3 mmol). ‘H NMR (400MHz, chloroform-d) ? 7.70 (s, 1H), 4.55 (qd, J=7.1, 1.1 Hz, 2H), 1.46 (td, J=7.2, 0.9 Hz, 3H).
 |
|
|