| Identification | Back Directory | [Name]
Carbamic acid, [(1S,3S)-3-aminocyclopentyl]-, 1,1-dimethylethyl ester (9CI) | [CAS]
645400-44-8 | [Synonyms]
[(1S,3S)-3-Amino-1-(boc-amino)cyclopentane
tert-butyl ((1S,3S)-3-aminocyclopentyl)carbamate
tert-butyl N-[(1S,3S)-3-aminocyclopentyl]carbamate
Carbamic acid, N-[(1S,3S)-3-aminocyclopentyl]-, 1,1-dimethylethyl ester
Carbamic acid, [(1S,3S)-3-aminocyclopentyl]-, 1,1-dimethylethyl ester (9CI) | [Molecular Formula]
C10H20N2O2 | [MDL Number]
MFCD11877864 | [MOL File]
645400-44-8.mol | [Molecular Weight]
200.28 |
| Chemical Properties | Back Directory | [Boiling point ]
304.2±31.0 °C(Predicted) | [density ]
1.04±0.1 g/cm3(Predicted) | [storage temp. ]
Keep in dark place,Inert atmosphere,Room temperature | [pka]
12.37±0.40(Predicted) | [InChI]
InChI=1S/C10H20N2O2/c1-10(2,3)14-9(13)12-8-5-4-7(11)6-8/h7-8H,4-6,11H2,1-3H3,(H,12,13)/t7-,8-/m0/s1 | [InChIKey]
PGBVMVTUWHCOHX-YUMQZZPRSA-N | [SMILES]
C(OC(C)(C)C)(=O)N[C@H]1CC[C@H](N)C1 |
| Hazard Information | Back Directory | [Uses]
tert-butyl N-[(1S,3S)-3-aminocyclopentyl]carbamate is used as a reactant in the synthesis of C-2 hydroxyethyl imidazopyrrolo pyridines as JAK1 inhibitors. | [Synthesis]
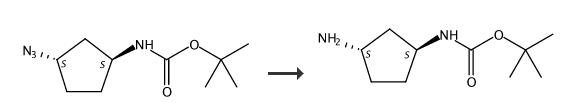
A flask containing tert-butyl [(1S,3S)-3-aminocyclopentyl]carbamate (16.5 g, crude ~0.05 mol) and 1.7 g Pd-C (10% paste) in MeOH (300 mL) was exposed to a positive pressure of hydrogen gas (balloon) over weekend. The catalyst was filtered off and the mixture was concentrated to afford the title compound (9.5 g) as a thick colorless viscous oil. Tert-butyl [(1S,3S)-3-aminocyclopentyl]carbamate, Yield (9.5 g) |
|
|