| Identification | Back Directory | [Name]
Berberine hydrochloride | [CAS]
633-65-8 | [Synonyms]
BBR
CI 75160
Kyoberin
NSC 646666
UMBELLATINE
BERBERIN HCL
BERBERINE HCL
Berberdine HCl
NATURAL YELLOW 18
TIMTEC-BB SBB006488
berberiniumchloride
C.I.Natural Yellow 18
BERBERINECHLORIDE(SH)
Berberine Chloride, JP
LABOTEST-BB LT00440956
BERBERINE CHLORIDE(AHP)
Berberine chloride form
BERBERINE HYDROCHLORIDE
Berberine chloride ,98%
Berberine chloride (TN)
Berberine Chloride (50 mg)
NATURAL YELLOW 18 CHLORIDE
Berberin chloride dihydrate
BERBERINE HYDROCHLORIDE N-HYDRATE
Berberine hydrochloride hydrate,97%
Berberine chloride form,Natural Yellow18
Berberinehydrochloride�����,Berberine chloride form
3)benzodioxolo(5,6-a)quinolizinium,5,6-dihydro-9,10-dimethoxy-benzo(g)(c
7,8,13,13a-tetradehydro-9,10-dimethoxy-2,3-(methylenedioxy)-berbiniuchlori
7,8,13,13a-Tetradehydro-9,10-diMethoxy-2,3-(Methylenedioxy)berbiniuM Chloride
9,10-Dimethoxy-5,6-dihydrobenzo[g]-1,3-benzodioxolo[5,6-a]quinolizinium·chloride
9,10-Dimethoxy-5,6-dihydro-benzo[g]-1,3-benzodioxolo[5,6-a]quinolizinium·chloride
Berbinium, 7,8,13,13a-tetradehydro-9,10-dimethoxy-2,3-(methylenedioxy)-, chloride
5,6-DIHYDRO-9,10-DIMETHOXY-BENZO[G]-1,3-BENZODIOXOLO[5,6-A]QUINOLIZINIUM, CHLORIDE
Benzo(g)(1,3)benzodioxolo(5,6-a)quinolizinium, 5,6-dihydro-9,10-dimethoxy-, chloride
9,10-Dimethoxy-5,6-dihydro-[1,3]dioxolo[4,5-g]isoquinolino[3,2-a]isoquinolin-7-ium chloride | [EINECS(EC#)]
211-195-9 | [Molecular Formula]
C20H18ClNO4 | [MDL Number]
MFCD00011939 | [MOL File]
633-65-8.mol | [Molecular Weight]
371.81 |
| Chemical Properties | Back Directory | [Appearance]
yellow crystalline powder | [Melting point ]
204-206 °C (dec.)
| [storage temp. ]
+2C to +8C | [solubility ]
methanol: soluble | [Colour Index ]
75160 | [form ]
Yellow powder | [color ]
Yellow powder | [Water Solubility ]
SOLUBLE IN COLD WATER | [BRN ]
3836585 | [Stability:]
Hygroscopic | [InChI]
InChI=1S/C20H18NO4.ClH/c1-22-17-4-3-12-7-16-14-9-19-18(24-11-25-19)8-13(14)5-6-21(16)10-15(12)20(17)23-2;/h3-4,7-10H,5-6,11H2,1-2H3;1H/q+1;/p-1 | [InChIKey]
VKJGBAJNNALVAV-UHFFFAOYSA-M | [SMILES]
C12C=C3C(C(OC)=C(OC)C=C3)=C[N+]=1CCC1=CC3=C(OCO3)C=C21.[Cl-] | [CAS DataBase Reference]
633-65-8 | [EPA Substance Registry System]
Benzo[g]-1,3-benzodioxolo[ 5,6-a]quinolizinium, 5,6-dihydro-9,10-dimethoxy-, chloride(633-65-8) |
| Hazard Information | Back Directory | [Chemical Properties]
yellow crystalline powder | [Uses]
Berberine hydrochloride(633-65-8): antiarrhythmic, alpha2 agonist, anticonvulsant, antiinflammatory, antibacterial, antifungal, antitrypanosomal, antineoplastic, immunostimulant
| [Uses]
An ingredient in some commercial eyewash products. | [Uses]
An isoqinoline alkaloid shown to have a chemopreventive property against colon tumor formation by inhibiting the enzyme cyclooxygenase-2 (cox-2) which is abundantly expressed in colon cancer cells. Al
so inhibits Activator Protein 1 (AP-1), a transcription factor that plays a critical role in inflammation and carcinogenesis. Treatment with berberine potentially results in the reduced accumulation o
f chemotherapeutic drugs. | [Definition]
ChEBI: Berberine chloride (TN) is an organic molecular entity. | [Biological Functions]
Although the mechanisms of action through which berberine exerts its effects are not yet fully elucidated, upon administration this agent appears to suppress the activation of various proteins and/or modulate the expression of a variety of genes involved in tumorigenesis and inflammation, including, but not limited to transcription factor nuclear factor-kappa B (NF-kB), myeloid cell leukemia 1 (Mcl-1), B-cell lymphoma 2 (Bcl-2), B-cell lymphoma-extra large (Bcl-xl), cyclooxygenase (COX)-2, tumor necrosis factor (TNF), interleukin (IL)-6, IL-12, inducible nitric oxide synthase (iNOS), intercellular adhesion molecule-1 (ICAM-1), E-selectin, monocyte chemoattractant protein-1 (MCP-1), C-X-C motif chemokine 2 (CXCL2), cyclin D1, activator protein (AP-1), hypoxia-inducible factor 1 (HIF-1), signal transducer and activator of transcription 3 (STAT3), peroxisome proliferator-activated receptor (PPAR), arylamine N-acetyltransferase (NAT), and DNA topoisomerase I and II. The modulation of gene expression may induce cell cycle arrest and apoptosis, and inhibit cancer cell proliferation. In addition, berberine modulates lipid and glucose metabolism. | [General Description]
A highly potent and selective oxysterol EBI2 (GPR183) agonist (Kd) = 450 pM in a saturation binding assay, and EC50 = 140 pM over EC50 = 2.1 nM for its enantiomer, 7β,25-OHC, in a GTP-γS binding assay). Dose-dependently suppresses forskolin-induced cAMP accumulation in an EBI2-expressing SK-N-MC/CRE-β-galactosidase cell line (IC50 = 2 nM), but not in control cells. Stimulates migration of LPS-activated spleen B-cells and anti-CD3/CD28-activated CD4+ T-cells in a dose-dependent manner. In addition, pharmacological inhibition of its biosynthesis in vivo by Clotrimazole, a CYP7B1inhibitor, promotes the migration of adoptively transferred pre-activated B cells to the T/B boundary, mimicking the phenotype of pre-activated B cells in EBI2-deficient mice. | [Biochem/physiol Actions]
An alkaloid with weak antibiotic properties. Substrate for MDR efflux pumps. Antimicrobial activities of berberine is potentiated by the MDR inhibitor 5′-methoxyhydnocarpin (5′-MHC). Berberine upregulates the expression of Pgp in hepatoma cells. Treatment with berberine potentially results in the reduced accumulation of chemotherapeutic drugs. | [Side effects]
Some side effects of berberine have been reported in research studies, primarily gastrointestinal symptoms such as nausea, abdominal pain, bloating, constipation, or diarrhea.
Berberine may interact with medicines. For example, it has been shown to interact with cyclosporine, a drug used to prevent rejection of transplanted organs. If you take medicine, talk with your health care provider if you consider taking berberine supplements.
Exposure to berberine has been linked to a harmful buildup of bilirubin in infants, which can cause brain damage. Therefore, berberine is likely to be unsafe for infants and may also be unsafe for use during pregnancy or while breastfeeding because of possible effects on the fetus or infant.
| [Safety Profile]
Poison by intraperitoneal route.Slightly toxic by ingestion. Mutation data reported. Whenheated to decomposition it emits toxic vapors of NOx andCl-. | [storage]
Store at 2-8°C | [Purification Methods]
Berberine chloride crystallises from water to give the dihydrate. The anhydrous salt may be obtained by recrystallisation from EtOH/Et2O, wash the crystals with Et2O and dry them in a vacuum. The iodide has m 250o(dec) (from EtOH). [Perkin J Chem Soc 113 503 1918, Kametani et al. J Chem Soc(C) 2036 1969, Beilstein 27 I 515, 27 II 567.] | [Clinical claims and research]
People most commonly use berberine for diabetes, high levels of cholesterol or other fats in the blood, and high blood pressure. It is also used for burns, canker sores, liver disease, and many other conditions but there is no good scientific evidence to support many of these uses. |
| Safety Data | Back Directory | [Hazard Codes ]
Xn | [Risk Statements ]
20/21/22-36/37/38 | [Safety Statements ]
24/25-36-26 | [RIDADR ]
1544 | [WGK Germany ]
2
| [RTECS ]
DR9866400
| [F ]
3-10 | [TSCA ]
Yes | [HazardClass ]
6.1(b) | [PackingGroup ]
III | [HS Code ]
29349990 | [Reaction]
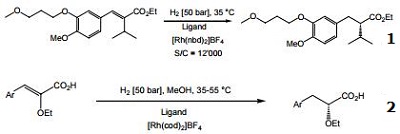
- Ligand used for the Rh-catalyzed asymmetric hydrogenation of α-substituted acrylic acids.
- Ligand used for the Rh-catalyzed asymmetric hydrogenation of 3-aryl-2-ethoxy-acrylic acids.
| [Toxicity]
LD50 orl-rat: >15 g/kg KSRNAM 8,654,74 |
|
|