| Identification | Back Directory | [Name]
5-BROMO-1H-INDAZOL-3-AMINE | [CAS]
61272-71-7 | [Synonyms]
3-Amino-5-bromoindazole
5-BROMO-1H-INDAZOL-3-AMINE
3-Amino-5-bromo-1H-indazole
5-BROMO-1H-INDAZOL-3-YLAMINE
1H-Indazol-3-amine, 5-bromo-
5-Bromo-1H-indazol-3-amine 95%
5-bromo-1H-indazol-3-amine (en)
3-Amino-5-bromo-1H-indazole 95%
3-Amino-5-bromo-1H-indazole,97%
5-bromo-1H-indazol-3-amine(SALTDATA: FREE)
ethyl 4-(aminomethyl)tetrahydro-2H-pyran-4-carboxylate
3-Amino-5-bromo-1H-indazole
5-Bromo-1H-indazol-3-amine | [Molecular Formula]
C7H6BrN3 | [MDL Number]
MFCD03426696 | [MOL File]
61272-71-7.mol | [Molecular Weight]
212.05 |
| Chemical Properties | Back Directory | [Melting point ]
171-175 | [Boiling point ]
431.3±25.0 °C(Predicted) | [density ]
1.867±0.06 g/cm3(Predicted) | [storage temp. ]
Room temperature. | [form ]
Liquid | [pka]
13?+-.0.40(Predicted) | [color ]
Clear colorless | [CAS DataBase Reference]
61272-71-7 |
| Hazard Information | Back Directory | [Uses]
5-BROMO-1H-INDAZOL-3-AMINE can be used as organic synthesis intermediate and pharmaceutical intermediate, mainly used in laboratory research and development process and chemical production process. | [Synthesis]
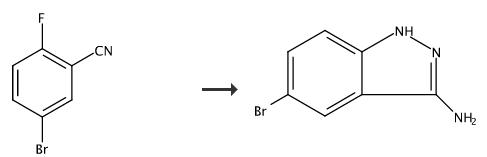
Hydrazine hydrate (18 mL) was added to Example 62C (1.93 g, 6.94 mmol.) in ethanol (10 mL). The mixture was heated to 95??C. overnight. The mixture was diluted with ethyl acetate and washed with water. Some of the product precipitated in the separatory funnel and was filtered to afford the title compound. The ethyl acetate layer was concentrated under reduced pressure and the resulting solid was triturated with methanol. 5-bromo-1H-indazol-3-amine. The title compound was prepared according to the procedure outlined in Example 62D substituting 5-bromo-2-fluorobenzonitrile for Example 62C. 1H NMR (400 MHz, DMSO-d6) |? ppm 11.55 (s, 1H), 7.92 (d, J=1.87 Hz, 1H), 7.30 (dd, J=8.79, 1.89 Hz, 1H), 7.19 (d, J=8.78 Hz, 1H), 5.41 (s, 2H). |
|
|