| Identification | Back Directory | [Name]
Binimetinib | [CAS]
606143-89-9 | [Synonyms]
MEK162
CS-394
ARRY 162
ARRY-162
ARRY-438162
Binimetinib
MEK162, ARRY-162
MEK162(Binimetinib)
MEK162 (ARRY-438162)
Binimetinib (MEK-162)
Binimetinib USP/EP/BP
Binimetinib ( Mektovi
Binimetinib(Free Base)
BINIMETINIB, ARRY-438162
Binimetinib,MEK162, ARRY-162
MEK162 (ARRY-162, ARRY-438162)
MEK162 (ARRY-438162,Binimetinib)
BiniMetinib (MEK162, ARRY-162, ARRY-438162)
1H-Benzimidazole-6-carboxamide,5-[(4-bromo-2-fluorophenyl)am...
ARRY 162; ARRY 438162; MEK-162;BINIMETINIB;MEK-162; ARRY-162; ARRY-438162
5-[(4-Bromo-2-fluorophenyl)amino]-4-fluoro-N-(2-hydroxyethoxy)-1-methyl-1H-benzimidazole-6-car
5-[(4-Bromo-2-fluorophenyl)amino]-4-fluoro-N-(2-hydroxyethoxy)-1-methylbenzimidazole-6-carboxamide
5-[(4-Bromo-2-fluorophenyl)amino]-4-fluoro-N-(2-hydroxyethoxy)-1-methyl-1H-benzimidazole-6-carboxamide
1H-Benzimidazole-6-carboxamide, 5-[(4-bromo-2-fluorophenyl)amino]-4-fluoro-N-(2-hydroxyethoxy)-1-methyl-
5-((4-bromo-2-fluorophenyl)amino)-4-fluoro-N-(2-hydroxyethoxy)-1-methyl-1H-benzo[d]imidazole-6-carboxamide
ARRY-438162
5-[(4-Bromo-2-fluorophenyl)amino]-4-fluoro-N-(2-hydroxyethoxy)-1-methyl-1H-benzimidazole-6-carboxamide
5-[(4-Bromo-2-fluorophenyl)amino]-4-fluoro-N-(2-hydroxyethoxy)-1-methyl-1H-benzimidazole-6-carboxamide Binimetinib (MEK162, ARRY-162, ARRY-438162) | [Molecular Formula]
C17H15BrF2N4O3 | [MDL Number]
MFCD22124525 | [MOL File]
606143-89-9.mol | [Molecular Weight]
441.227 |
| Chemical Properties | Back Directory | [Melting point ]
>203oC (dec.) | [density ]
1.67 | [storage temp. ]
-20°C | [solubility ]
Soluble in DMSO (up to at least 25 mg/ml) | [form ]
solid | [pka]
14.20±0.10(Predicted) | [color ]
White | [Stability:]
Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 3 months. |
| Questions And Answer | Back Directory | [Kinase inhibitor]
Binimetinib, also known as Mektovi, is a potent and selective oral mitogen-activated protein kinase 1/2 (MEK 1/2) inhibitor with potential antineoplastic activity.
Binimetinib, noncompetitive with ATP, binds to and inhibits the activity of MEK1/2. Inhibition of MEK1/2 prevents the activation of MEK1/2 dependent effector proteins and transcription factors, which may result in the inhibition of growth factor-mediated cell signaling. This may eventually lead to an inhibition of tumor cell proliferation and an inhibition in production of various inflammatory cytokines including interleukin-1, -6 and tumor necrosis factor.
| [Mechanism of Action]
Binimetinib is a reversible inhibitor of mitogen-activated extracellular signal regulated kinase 1 (MEK1) and MEK2 activity. MEK proteins are upstream regulators of the extracellular signal-related kinase (ERK) pathway. In vitro, binimetinib inhibited extracellular signal-related kinase (ERK) phosphorylation in cellfree assays as well as viability and MEK-dependent phosphorylation of BRAF-mutant human melanoma cell lines. Binimetinib also inhibited in vivo ERK phosphorylation and tumor growth in BRAF-mutant murine xenograft models. | [Pharmacokinetics]
The primary metabolic pathway is glucuronidation with UGT1A1 contributing up to 61% of the binimetinib metabolism. Other pathways of binimetinib metabolism include N-dealkylation, amide hydrolysis, and loss of ethane-diol from the side chain. The active metabolite M3 produced by CYP1A2 and CYP2C19 represents 8.6% of the binimetinib exposure. Following a single oral dose of 45 mg radiolabeled binimetinib, approximately 60% of the circulating radioactivity AUC in plasma was attributable to binimetinib. | [Binding Mode]
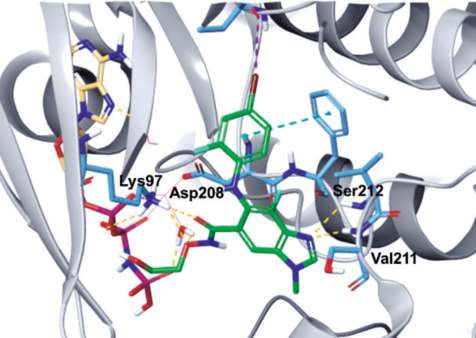
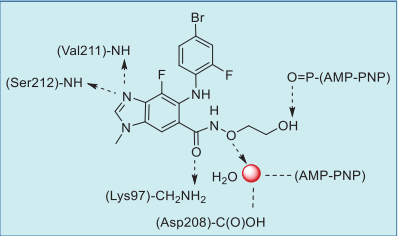
As shown in the co-crystal structure of
binimetinib in complex with BRAF–MEK1 kinases
and AMP–PNP (Fig. 1), the imine nitrogen of the
benzo[d]imidazole core hydrogen bonds to both the
amide NH of Ser212 and amide NH of Val211, and
the amide oxygen also forms a hydrogen bond with
the primary amine of Lys97. In addition, the terminal
hydroxyl group hydrogen bonds to the α-phosphate
oxygen of AMP–PNP. Also, the carboxamide side
chain oxygen interacts indirectly with the carboxylic
acid of Asp208 and AMP–PNP via a water molecule
(Fig. 2).
  |
| Hazard Information | Back Directory | [Description]
Binimetinib (606143-89-9) is a potent (IC50?= 12 nM) and selective allosteric inhibitor of MEK1/2.1,2?Recently approved by the FDA for treatment of melanoma in combination with Encorafenib. Binimetinib has had limited success as monotherapy but has shown promise in combination with other chemotherapeutic agents.3-5 | [Uses]
Binimetinib is a potent inhibitor of MEK1/2 with an IC50 of 12 nM in a cell-free assay. | [Definition]
ChEBI: Binimetinib is a member of the class of benzimidazoles that is 1-methyl-1H-benzimidazole which is substituted at positions 4, 5, and 6 by fluorine, (4-bromo-2-fluorophenyl)nitrilo, and N-(2-hydroxyethoxy)aminocarbonyl groups, respectively. It is a MEK1 and MEK2 inhibitor (IC50= 12 nM). Approved by the FDA for the treatment of patients with unresectable or metastatic melanoma with a BRAF V600E or V600K mutation in combination with encorafenib. It has a role as an EC 2.7.11.24 (mitogen-activated protein kinase) inhibitor, an antineoplastic agent and an apoptosis inducer. It is a member of benzimidazoles, a member of bromobenzenes, a member of monofluorobenzenes, a hydroxamic acid ester and a secondary amino compound. | [Brand name]
Mektovi | [General Description]
Class: dual threonine/tyrosine kinase;
Treatment: melanoma with BRAF mutations; Other name: ARRY-162; Oral bioavailability = 50%;
Elimination half-life = 3.5 h;
Protein binding = 97% | [Enzyme inhibitor]
This MEK inhibitor (FW = 441.23 g/mol; CAS 606143-89-9; Solubility: 88
mg/mL DMSO, when warmed), also named MEK162, ARRY-162, ARRY-
438162, and 5- ( (4-bromo-2-fluorophenyl) amino) -4-fluoro-N- (2-hydroxy-
ethoxy) -1-methyl-1H-benzo[d]imidazole-6-carboxamide, targets Mitogen-
Activated Protein Kinase Kinase (MAPKK), also known as MAP2K and
MEK, which phosphorylates Mitogen-Activated Protein Kinase (MAPK).
(IC50 = 12 nM) and is the first targeted therapy to show activity in patients
with NRAS -mutated melanoma. | [target]
MEK1 | [References]
1) Lee?et al.?(2010),?Preclinical development of ARRY-162, a potent and selective MEK1/2 inhibitor;?Cancer Res.?70?2515
2) Winski?et al.?(2010),?MEK162 (ARRY-162), a novel MEK ? inhibitor, inhibits tumor growth regardless of KRAS/RAF pathway mutations;?EJC Supplements?8?56
3) Lee?et al.?(2016),?Efficacy of the combination of MEK and CDK4/6 inhibitors in vitro and in vivo in KRAS mutant colorectal cancer models;?Oncotarget?7?39595
4) Gong?et al.?(2017),?MEK162 Enhances Antitumor Activity of 5-Fluorouracil and Trifluridine in KRAS-mutated Human Colorectal Cancer Cell Lines;?Anticancer Res.?37?2831
5) Van Cutsem?et al.?(2019),?Binimetinib, Encorafenib, and Cetuximab Triplet Therapy for Patients With BRAF V600E-Mutant Metastatic Colorectal Cancer: Safety Lead-In Results From Phase III BEACON Colorectal Cancer study;?J. Clin. Oncol.?180?2459 |
|
|