| Identification | Back Directory | [Name]
PROMETHAZINE | [CAS]
60-87-7 | [Synonyms]
Dimapp
Fargan
Procit
Prorex
WY 509
RP 3277
Tanidil
Phargan
Fenazil
Hiberna
3277 RP
A-91033
Aprobit
Avomine
Iergigan
Diprozin
Lercigan
Lergigan
Thiergan
SKF 1498
Synalgos
Provigan
Romergan
Protazine
Prothazin
Valergine
Phenargan
Phensedyl
Pilpophen
Pipolphen
Histargan
3389 R.P.
4182 R.P.
Diphergan
Diprazine
NSC 30321
Fenetazina
Prometasin
Prometazin
NCI-C60673
Lilly 1516
Vallergine
Proazamine
Lilly 01516
Promethazin
Proazaimine
Promethaine
PROMETHAZINE
Isophenergan
Promezathine
Pyrethiazine
Promazinamide
Promethiazine
Isopromethazine
PROMETHAZINE USP/EP/BP
Dimethylamino-isopropyl-phenthiazin
10-(2-DIMETHYLAMINOPROPYL)-PHENOTHIAZIN
Promethazine for peak identification CRS
10-[2-(Dimethylamino)propyl]phenothiazine
Promethazine (base and/or unspecified salts)
Phenothiazine, 10-[2-(dimethylamino)propyl]-
10-[2-(Dimethylamino)propyl]-10H-phenothiazine
N,N-dimethyl-1-phenothiazin-10-ylpropan-2-amine
N,N,a-Trimethyl-10H-phenothiazine-10-ethanamine
N-Dimethylamino-2-methylethyl thiodiphenylamine
N-(2'-Dimethylamino-2'-methyl)ethylphenothiazine
10H-Phenothiazine-10-ethanaMine,N,N,a-triMethyl-
N,N-dimethyl-1-phenothiazin-10-yl-propan-2-amine
10-(2-(Dimethylamino)-2-methylethyl)phenothiazine
10H-Phenothiazine-10-ethanamine, N,N,α-trimethyl-
Phenothiazine, 10-[2-(dimethylamino)propyl]- (8CI)
dimethyl-(1-methyl-2-phenothiazin-10-yl-ethyl)amine
N,N-diMethyl-1-(10H-phenothiazin-10-yl)propan-2-aMine
10H-Phenothiazine-10-ethanamine, N,N,alpha-trimethyl-
(2-Dimethylamino-2-methyl)ethyl-N-dibenzoparathiazine
N,N-Dimethyl-1-(10H-phenothiazin-10-yl)-2-propanamine
(Dimethylamino-2-propyl-10-phenothiazine hydrochloride
10H-Phenothiazine-10-ethanamine, N,N,a-trimethyl- (9CI) | [EINECS(EC#)]
200-489-2 | [Molecular Formula]
C17H20N2S | [MDL Number]
MFCD00066294 | [MOL File]
60-87-7.mol | [Molecular Weight]
284.42 |
| Hazard Information | Back Directory | [Uses]
Anti-emetic; antihistaminic. | [Definition]
ChEBI: A tertiary amine that is a substituted phenothiazine in which the ring nitrogen at position 10 is attached to C-3 of an N,N-dimethylpropan-2-amine moiety. | [Brand name]
Phenergan (Wyeth). | [General Description]
Crystals. Melting point 60°C. Used as an antihistaminic. | [Air & Water Reactions]
Turns blue on prolonged exposure to air and moisture. | [Reactivity Profile]
PROMETHAZINE neutralizes acids in exothermic reactions to form salts plus water. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable or toxic gases may be generated in combination with strong reducing agents, such as hydrides. | [Health Hazard]
SYMPTOMS: Symptoms of PROMETHAZINE include leucopenia; agranulocytosis; confusion; convulsions; stupor; and it potentiates the action of central nervous system depressants. | [Fire Hazard]
Flash point data for PROMETHAZINE are not available, however PROMETHAZINE is probably combustible. | [Description]
As a derivative of phenothiazine, promethazine is structurally and pharmacologically similar
to chlorpromazine. It exhibits strong antihistamine activity as well as expressed action
on the CNS. It potentiates action of sedative and analgesic drugs. | [World Health Organization (WHO)]
Introduced in 1946, promethazine, a phenothiazine derivative has
a variety of pharmacological properties. At present it is mainly used as an
antihistamine and anti-motion-sickness drug. Promethazine is listed in the WHO
Model List of Essential Drugs. | [Clinical Use]
Promethazine, an early agent in the series, has many useful pharmacological affects other
than being an antihistamine. It has significant antiemetic and anticholinergic properties. It
also has sedative-hypnotic properties and has been used to potentiate the effects of
analgesic drugs. Subsequent analogues, such as trimeprazine and methdilazine, are used as
antipruritic agents in the treatment of urticaria. | [Synthesis]
Promethazine, 10-(2-dimethylaminopropyl)phenothiazine (16.1.18), is
synthesized by alkylating phenothiazine with 1-dimethylamino-2-propylchloride.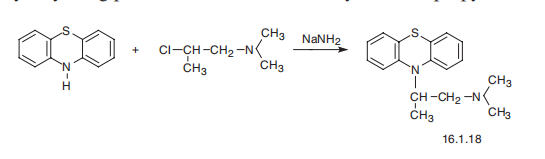
|
| Safety Data | Back Directory | [Safety Profile]
Poison by ingestion,
intravenous, intramuscular, intraperitoneal,
and subcutaneous routes. Human systemic
effects by ingestion: pupillary dilation,
wakefulness, hallucinations, and distorted
perceptions. An experimental teratogen.
Other experimental reproductive effectsHuman mutation data reported. A severe
eye irritant. When heated to decomposition
it emits very toxic fumes of NOx and SOx | [Hazardous Substances Data]
60-87-7(Hazardous Substances Data) | [Toxicity]
LD50 oral in rabbit: 580mg/kg |
|
|