| Identification | Back Directory | [Name]
Aclatonium napadisilate | [CAS]
55077-30-0 | [Synonyms]
Abovis
TM 723
Aclatonium
aclatonium napadisilate
aclatonium napadisylate
(2-Acetoxypropionyl)choline
Napadisilic acid aclatonium
Aclatonium napadisilate USP/EP/BP
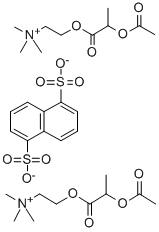
2-(2-acetyloxy-1-oxopropoxy)ethyl-trimethylammonium
2-[2-(Acetyloxy)-1-oxopropyloxy]-N,N,N-trimethylethanaminium
(2-acetyllactoyloxyethyl)trimethylammonium 1,5-naphthalenedisulfonate
(2-acetyllactoyloxyethyl)trimethylammonium hemi-1,5-naphthalenedisulfonate
2-(2-acetyloxypropanoyloxy)ethyl-trimethylazanium,naphthalene-1,5-disulfonate
Ethanaminium, 2-[2-(acetyloxy)-1-oxopropoxy]-N,N,N-trimethyl-, 1,5-naphthalenedisulfonate (2:1)
1,5-Naphthalenedisulfonic acid, ion(2-), bis[2-[2-(acetyloxy)-1-oxopropoxy]-N,N,N-trimethylethanaminium] (9CI)
2-(2-acetyloxypropanoyloxy)ethyl-[2-[2-(2-acetyloxypropanoyloxy)ethyl-dimethyl-ammonio]ethyl]-dimethyl-azanium naphthalene-1,5-disulfonate | [EINECS(EC#)]
1592732-453-0 | [Molecular Formula]
C30H44N2O14S2 | [MOL File]
55077-30-0.mol | [Molecular Weight]
720.805 |
| Hazard Information | Back Directory | [Originator]
Abovis ,Toyama ,Japan ,1981 | [Manufacturing Process]
5.2 g of bis(choline)-naphthalene-1,5-disulfonate was suspended in 30 ml of
acetonitrile, and 10 g of lactic acid anhydride diacetate was added thereto.
This mixture was refluxed for 3 hours. The resulting reaction mixture was
allowed to stand at room temperature while cooling to precipitate the desired
product crystals, which were collected by filtration. 5.5 g (76% yield) of the
desired product having a melting point of 189°C to 191°C were obtained. | [Therapeutic Function]
Cholinergic | [Safety Profile]
Poison by intravenous route.Moderately toxic by subcutaneous route. An experimentalteratogen. Other experimental reproductive effects. Whenheated to decomposition it emits toxic fumes of NOx andSOx. A cholinergic agent. |
|
| Company Name: |
LGM Pharma
|
| Tel: |
1-(800)-881-8210 |
| Website: |
www.lgmpharma.com |
|