| Identification | Back Directory | [Name]
2-METHYL-3,1-BENZOXAZA-4-ONE | [CAS]
525-76-8 | [Synonyms]
525-76-8
NSC 10119
NSC 521353
ACETANTHRANIL
AURORA KA-3785
Acetylanthranyl
ACETYL ANTHRANIL
TIMTEC-BB SBB000135
2-METHYL-3,1-BENZOXAZA-4-ONE
2-methyl-3,1-benzoxazin-4-one
2-methyl-4h-1-benzoxazin-4-one
2-Methylbenzo[1,3]oxazin-4-one
2-Methyl-3,1-benzoxazine-4-one
2-Methyl-4-oxo-3,1-benzoxazine
2-Methyl-4H-3,1-benzoxazin-4-o
2-METHYL-4H-3,1-BENZOXAZIN-4-ONE
2-Methyl-4-oxo-4H-3,1-benzoxazine
2-Methyl-benzo(d)(1,3)oxazin-4-one
2-2-METHYL-4H-3,1-BENZOXAZIN-4-ONE
4H-3,1-benzoxazin-4-one, 2-methyl-
2-Methyl-4H-3,1-benzoxazin-4-one 98%
2-Methyl-4H-benzo[d][1,3]oxazin-4-one
2-Methylbenzisoxazinone(2-Methyl-4H-3,1-Benzoxazin-4-One)
3-methyl-1-phenyl-6,7-dihydro-5H-pyrrolo[2,3-d]pyrimidine-2,4-dione | [EINECS(EC#)]
208-381-7 | [Molecular Formula]
C9H7NO2 | [MDL Number]
MFCD00047647 | [MOL File]
525-76-8.mol | [Molecular Weight]
161.16 |
| Chemical Properties | Back Directory | [Melting point ]
79-82 °C(lit.)
| [Boiling point ]
144-145 °C(Press: 12 Torr) | [density ]
1.25±0.1 g/cm3(Predicted) | [storage temp. ]
Inert atmosphere,2-8°C | [pka]
2.84±0.20(Predicted) | [InChI]
InChI=1S/C9H7NO2/c1-6-10-8-5-3-2-4-7(8)9(11)12-6/h2-5H,1H3 | [InChIKey]
WMQSKECCMQRJRX-UHFFFAOYSA-N | [SMILES]
N1C2=CC=CC=C2C(=O)OC=1C | [EPA Substance Registry System]
4H-3,1-Benzoxazin-4-one, 2-methyl- (525-76-8) |
| Hazard Information | Back Directory | [Uses]
2-Methyl-4H-3,1-benzoxazin-4-one is a synthetic antimicrobial agent. It belongs to the quinazolinone class of drugs. It has been shown to be effective against Staphylococcus aureus and other bacteria. Quinazolinones are potent inhibitors of bacterial growth and have been used successfully in the treatment of tuberculosis and other infectious diseases. 2-Methyl-4H-3,1-benzoxazin-4-one has also been shown to inhibit the growth of some Gram-negative bacteria, such as Salmonella typhi and Escherichia coli. | [Synthesis Reference(s)]
The Journal of Organic Chemistry, 45, p. 363, 1980 DOI: 10.1021/jo01290a038 | [Synthesis]
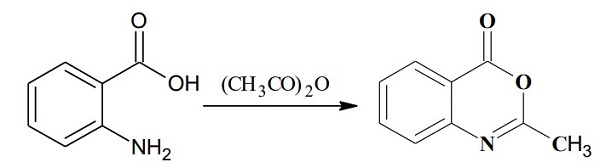
In 250 mL of RBF took (0.01 mole or 1.37 gm) anthranilic acid (2-amino benzoic acid) to that added 15 mL of acetic anhydride (excess) with porcelain chips. Reflux the solution at 35-40 ℃ for 50-55 min on heating mantle. TLC was done using EA: n-hexane (1:1) solvent system. Then removed the excess solvent (acetic anhydride) at low pressure using rotatory evaporator, To the remaining solid, added petroleum ether (40-60) to extract the 2-methyl-4H-3,1-benzoxazin-4-one. Repeated petroleum ether step to extract the entire product. After drying of petroleum-ether, the crystals of 2-methyl-4H-3, 1-benzoxazin-4-one was obtained and then it was recrystallized with ethanol8. M.P 80-82℃, Yield 79 %.
 |
|
|