| Identification | Back Directory | [Name]
ReMogliflozin etabonate | [CAS]
442201-24-3 | [Synonyms]
EOS-61714
GSK 189075
ReMogliflozin etabonate
UAOCLDQAQNNEAX-ABMICEGHSA-N
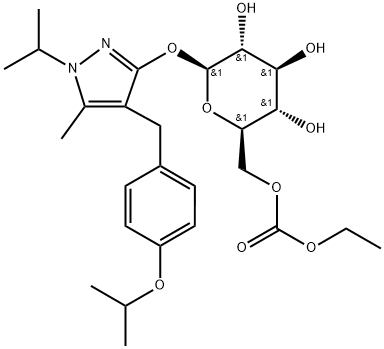
β-D-Glucopyranoside, 5-methyl-4-[[4-(1-methylethoxy)phenyl]methyl]-1-(1-methylethyl)-1H-pyrazol-3-yl, 6-(ethyl carbonate)
ethyl [(2R,3S,4S,5R,6S)-3,4,5-trihydroxy-6-[5-methyl-1-propan-2-yl-4-[(4-propan-2-yloxyphenyl)methyl]pyrazol-3-yl]oxyoxan-2-yl]methyl carbonate
ethyl(((2R,3S,4S,5R,6S)-6-((4-(4-isopropoxybenzyl)-1-isopropyl-5-methyl-1H-pyrazol-3-yl)oxy)-3,4,5-tri(l1-oxidanyl)tetrahydro-2H-pyran-2-yl)methyl)carbonateGSK-189075 | [Molecular Formula]
C26H38N2O9 | [MDL Number]
MFCD17167013 | [MOL File]
442201-24-3.mol | [Molecular Weight]
522.6 |
| Chemical Properties | Back Directory | [Boiling point ]
661.3±55.0 °C(Predicted) | [density ]
1.31±0.1 g/cm3(Predicted) | [storage temp. ]
Store at -20°C | [solubility ]
DMSO: Soluble; Methanol: Soluble | [form ]
A solid | [pka]
12.58±0.70(Predicted) | [color ]
White to off-white |
| Hazard Information | Back Directory | [Uses]
Remogliflozin etabonate (GSK189075) is an orally active, selective and low-affinity sodium glucose cotransporter (SGLT2) inhibitor with Ki values of 1.95 μM, 2.14 μM, 43.1 μM, 8.57 μM for hSGLT2, rSGLT2, hSGLT1, rSGLT1, respectively. Remogliflozin etabonate is a proagent based on benzylpyrazole glucoside and is metabolized to its active form, Remogliflozin, in the body. Remogliflozin etabonate exhibits antidiabetic efficacy in rodent models[1]. | [Definition]
ChEBI: Remogliflozin etabonate is a glycoside. | [in vivo]
Remogliflozin etabonate (GSK189075; 10 or 30 mg/kg; orally; twice daily for 6 weeks) significantly reduced both the FPG and GHb levels in a dosedependent manner[1].
Remogliflozin etabonate (3, 10, 30 mg/kg; orally) increases urinary glucose excretion in a dose-dependent manner. Remogliflozin etabonate dose-dependently inhibits the increase in plasma glucose after glucose loading and decreases the plasma insulin in normal rats[1].
Remogliflozin etabonate (1-10 mg/kg; orally) decreases the blood glucose and reduces the AUC0–6 h for blood glucose in a dose-dependent manner[1].
Remogliflozin etabonate (high-fat diet containing 0.01, 0.03, or 0.1% remogliflozin etabonate for 8 weeks) reduces the levels of plasma glucose, plasma insulin, and GHb in a dose-dependent manner, and it suppresses the development of hypertriglyceridemia in male GK rats (6 weeks of age)[1].
| Animal Model: | db/db mice at the age of 8 weeks[1] | | Dosage: | 10 or 30 mg/kg | | Administration: | Orally; twice daily for 6 weeks | | Result: | Significantly reduced both the fasting plasma glucose (FPG) and glycated hemoglobin (GHb) levels in a dosedependent manner.
Reduced both urine volume and urinary glucose excretion with ameliorated the hyperglycemia.
|
| [IC 50]
hSGLT2: 1.95 μM (Ki); rSGLT2: 2.14 μM (Ki); hSGLT1: 43.1 μM (Ki); rSGLT1: 8.57 μM (Ki) | [References]
[1] Yoshikazu Fujimori, et al. Remogliflozin Etabonate, in a Novel Category of Selective Low-Affinity Sodium Glucose Cotransporter (SGLT2) Inhibitors, Exhibits Antidiabetic Efficacy in Rodent Models. J Pharmacol Exp Ther. 2008 Oct;327(1):268-76. DOI:10.1124/jpet.108.140210 |
|
| Company Name: |
LGM Pharma
|
| Tel: |
1-(800)-881-8210 |
| Website: |
www.lgmpharma.com |
|