| Identification | Back Directory | [Name]
Imrecoxib | [CAS]
395683-14-4 | [Synonyms]
Imrecoxib
Imrecoxib USP/EP/BP
2H-Pyrrol-2-one, 1,5-dihydro-3-(4-methylphenyl)-4-[4-(methylsulfonyl)phenyl]-1-propyl- | [Molecular Formula]
C21H23NO3S | [MOL File]
395683-14-4.mol | [Molecular Weight]
369.48 |
| Chemical Properties | Back Directory | [Boiling point ]
604.1±55.0 °C(Predicted) | [density ]
1?+-.0.06 g/cm3(Predicted) | [storage temp. ]
Store at -20°C | [solubility ]
DMSO: 100 mg/mL (270.65 mM) | [form ]
Solid | [pka]
-2.25±0.60(Predicted) | [color ]
White to off-white | [CAS DataBase Reference]
395683-14-4 |
| Hazard Information | Back Directory | [Uses]
Imrecoxib (BAP-909) is a novel and selective cyclooxygenase 2 (COX-2) inhibitor with an IC50 value of 18 nM, it also inhibits COX1- activity with an IC50 value of 115 nM. Imrecoxib (BAP-909) has anti-inflammatory effect[1]. | [Clinical Use]
Imrecoxib, a new non-steroid anti-inflammtory drug (NSAID), was launched in China with the trade
name of Hengyang® for the treatment of osteoarthritis in 2012. It was originally designed and
synthesized by Guo and co-workers at the Institute of Materia Medica (IMM) of the Chinese Academy
of Medical Sciences in collaboration with Hengrui Pharmaceuticals. Imrecoxib, which is a moderately
selective COX-2 inhibitor (with IC50 values against COX-1 and COX-2 being 115 ± 28 nM and 18 4
nM, respectively). | [Synthesis]
Imrecoxib has had two synthetic routes reported across several publications. The most likely process-scale route to this drug is described in the scheme, which began with 2-bromo-4'-(methylsulfonyl)-acetophenone (84) and p-tolylacetic acid (85) as starting materials. In the
presence of base, |á-bromoketone 84 was treated with acid 85 which resulted in lactone 86 in 72% yield
across the two-step sequence. Exposure of lactone 86 with propylamine triggered a ring-opening-ring
closing reaction, which resulted in imrecoxib (XIII) directly in 85% yield.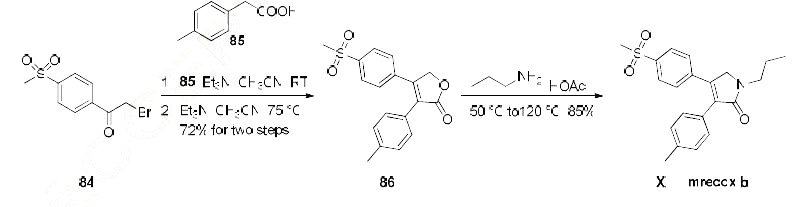
| [in vivo]
Imrecoxib (BAP-909) (gastrointestinal administration; 5-20 mg/kg; 1 hour before carrageenan injection) inhibits carrageenan-induced acute inflammation, and the inhibitory effect is maximal at 4 hours[1].
Imrecoxib (BAP-909) (gastrointestinal administration; 5-20 mg/kg; started on day 7; 26 days) diminishes the secondary paw swelling and inhibits heat-inactivated BCG induced-inflammtory polyarthritis[1]. | Animal Model: | Rat carrageenan-induced edema model[1] | | Dosage: | 5 mg/kg, 10 mg/kg, 20 mg/kg | | Administration: | Gastrointestinal administration; 5-20 mg/kg; 1 hour before carrageenan injection | | Result: | Inhibited the edema response with different doses. |
| Animal Model: | Rat adjuvant-induced arthritis (AIA) model[1] | | Dosage: | 5 mg/kg, 10 mg/kg, 20 mg/kg | | Administration: | Gastrointestinal administration; 5-20 mg/kg; started on day 7; 26 days | | Result: | Inhibited adjuvant-induced chronic inflammation at the doses of 10 and 20 mg/kg. |
| [IC 50]
Human COX-1: 115 nM (IC50); Human COX-2: 18 nM (IC50) | [storage]
Store at -20°C | [References]
[1] Chen XH, et al. Imrecoxib: a novel and selective cyclooxygenase 2 inhibitor with anti-inflammatory effect. Acta Pharmacol Sin. 2004 Jul;25(7):927-31. PMID:15210067 |
|
|