| Identification | Back Directory | [Name]
Pirbuterol | [CAS]
38677-81-5 | [Synonyms]
Pirbuterol
2-tert-Butylamino-1-(5-hydroxy-6-hydroxymethyl-2-pyridyl)ethanol
6-[2-(tert-Butylamino)-1-hydroxyethyl]-3-hydroxy-2-pyridinemethanol
6-[2-(tert-butylamino)-1-hydroxyethyl]-2-(hydroxymethyl)pyridin-3-ol
2,6-Pyridinedimethanol, a6-[[(1,1-dimethylethyl)amino]methyl]-3-hydroxy- (9CI) | [Molecular Formula]
C12H20N2O3 | [MDL Number]
MFCD00867058 | [MOL File]
38677-81-5.mol | [Molecular Weight]
240.3 |
| Hazard Information | Back Directory | [Brand name]
Maxair (3M Pharmaceuticals). | [Description]
Pirbuterol is a β2-agonist in the
management of disorders with reversible airways
obstruction. It is given by inhalation and
orally. | [Originator]
Exirel ,Pfizer Taito ,Japan,1982 | [Uses]
Bronchodilator. | [Uses]
This relatively selective β2-adrenergic receptor agonist is structurally very similar to
albuterol, and it displays similar broncholytic properties. It is used as an inhaled drug for
treating bronchial asthma. | [Definition]
ChEBI: Pirbuterol is a member of pyridines. | [Manufacturing Process]
To 78 ml of a 1 M solution of diborane in tetrahydrofuran under nitrogen and cooled to 0°C is added dropwise over a period of 40 minutes 13.5 g of N-tertbutyl-2-(5-benzyloxy-6-hydroxymethyl-2-pyridyl)-2-hydroxyacetamide in 250 ml of the same solvent. The reaction mixture is allowed to stir at room temperature for 3.5 hours, and is then heated to reflux for 30 minutes and cooled to room temperature. Hydrogen chloride (70 ml, 1.34 N) in ethanol is added dropwise, followed by the addition of 300 ml of ether. The mixture is allowed to stir for 1 hour and is then filtered, yielding 11.0 g, melting point 202°C (dec.). The hydrochloride dissolved in water is treated with a sodium hydroxide solution to pH 11 and is extracted into chloroform (2 x 250 ml). The chloroform layer is dried over sodium sulfate, concentrated to dryness in vacuo, and the residue recrystallized from isopropyl ether, 3.78 g, melting point 81°C to 83.5°C.
A solution of 1.7 g of 2-hydroxymethyl-3-benzyloxy-(1-hydroxy-2-tert-butylaminoethyl)pyridine in 30 ml of methanol containing 1.2 ml of water is shaken with 700 mg of 5% palladiumon-charcoal in an atmosphere of hydrogen at atmospheric pressure. In 17 minutes the theoretical amount of hydrogen has been consumed and the catalyst is filtered. Concentration of the filtrate under reduced pressure provides 1.4 g of the crude product as an oil. Ethanol (5 ml) is added to the residual oil followed by 6 ml of 1.75 N ethanolic hydrogen chloride solution and, finally, by 5 ml of isopropyl ether. The precipitated product is filtered and washed with isopropyl ether containing 20% ethanol, 1.35 g, melting point 182°C (dec.). | [Therapeutic Function]
Bronchodilator | [Synthesis]
Pirbuterol, |á(6)¨C[[[1,1-dimethylethyl]amino]methyl]-3-hydroxy-2,6-pyridindimethanol
(23.3.22), is synthesized from 3-hydroxypyridine, which undergoes subsequent
hydroxymethylation and further alkylation by benzylchloride at the aromatic
hydroxyl group, giving 3-benzyloxy-2,6-bis-(hydroxymethyl)pyridine (23.3.17). Selective
oxidation of the 6-hydroxymethyl group using manganese peroxide gives 3-benzyloxy-2-
hydroxymethylpiperidine-6-aldehyde (23.3.18). Condensation of this with nitromethane
gives the corresponding nitromethylcarbinol 23.3.19, the nitro group of which is reduced
to an amine group by hydrogen using Raney nickel as a catalyst, which forms an aminoalcohol
23.3.20. Alkylation of the aminogroup with tert-butylbromide gives a secondary
amine (23.3.21), and removing the protective benzyl group by hydrogen reduction forms
pirbuterol (23.3.22) .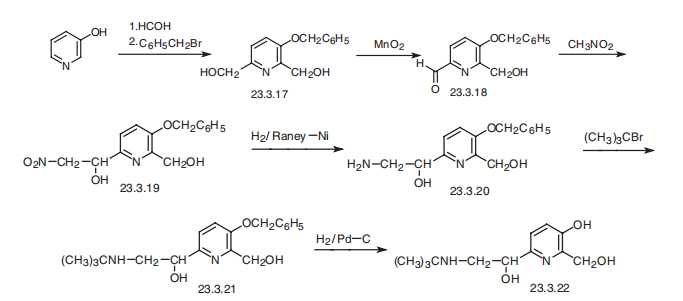
|
|
| Company Name: |
cjbscvictory
|
| Tel: |
13348960310 13348960310 |
| Website: |
https://www.weikeqi-biotech.com/ |
|