| Identification | Back Directory | [Name]
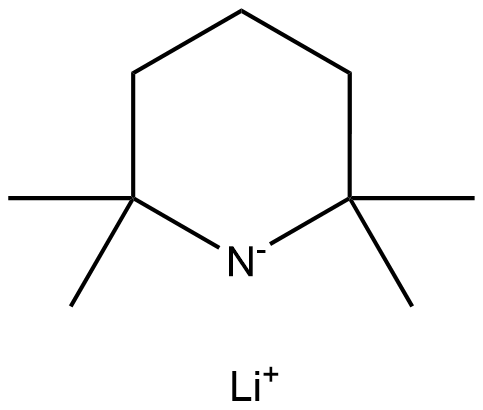
LITHIUM TETRAMETHYLPIPERIDIDE | [CAS]
38227-87-1 | [Synonyms]
LTMP
LiTMP
piperidide
ithium 2,2,6,6-tetramethyL
LITHIUM TETRAMETHYLPIPERIDIDE
Lithium·2,2,6,6-tetramethylpiperidine
LithiuM 2,2,6,6-tetraMethylpiperidide
"2,2,6,6-tetramethylpiperidine lithium"
LithiuM 2,2,6,6-tetraMethylpiperidide 97%
Lithium 2,2,6,6-tetramethylpiperidin-1-ide
Piperidine, 2,2,6,6-tetramethyl-, lithium salt
2,2,6,6-Tetramethylpiperidine lithium salt (1:1)
Piperidine,2,2,6,6-tetramethyl-, lithium salt (1:1) | [EINECS(EC#)]
684-475-5 | [Molecular Formula]
C9H18LiN | [MDL Number]
MFCD00015910 | [MOL File]
38227-87-1.mol | [Molecular Weight]
147.19 |
| Hazard Information | Back Directory | [Physical properties]
Lithium 2,2,6,6-Tetramethylpiperidide exists in THF solution as a dimer-monomer equilibrium mixture; additives such as HMPA
increase monomer concentration. X-ray structure determination shows that LiTMP crystallizes as a
tetramer from hexane/pentane mixtures. | [Uses]
Lithium 2,2,6,6-tetramethylpiperidide is a strong base and can be used:
- For the synthesis of enamines from terminal epoxides through trans-α-lithiated epoxide as an intermediate.
- For ortholithiation of arenes such as 1,3-bis(trifluoromethyl)benzene, 4,4-dimethyl-2-phenyl-2-oxazoline, 1,4-bis(trifluoromethyl)benzene and 1,3-dimethoxybenzene.
- In combination with Lewis donor ligand, N,N,N′,N′-tetramethylethylenediamine (TMEDA) for deprotonative metalation of methoxy-substituted arenes.{21]
| [Application]
Lithium 2,2,6,6-Tetramethylpiperidide (LTMP) is a strong, highly hindered, nonnucleophilic base (pKa = 37.3) capable of selective deprotonation of aromatics,
heteroaromatics, and aliphatic C-H acidic sites in the presence of a variety of functional groups; also
compatible with several electrophiles for in situ quenching of kinetically derived lithiated species. | [Preparation]
Preparative Method of Lithium 2,2,6,6-Tetramethylpiperidide: prepared in Et2O or THF solutions immediately before use by treatment of
commercially available dry 2,2,6,6-Tetramethylpiperidine with n-Butyllithium (1:1). Tetramethylpiperidine
is dried by heating a mixture of the base with CaH2 at reflux for 4 h in a preflamed flask, followed by
distillation at atmospheric pressure (bp 152 °C). It should be stored in a septum sealed bottle. | [storage]
LiTMP solutions are pyrophoric, can cause severe burns, and should
always be handled and transferred under an inert atmosphere. Solutions of LiTMP show a loss of activity
(50% in THF; 60% in Et2O) after 12 h at 24 °C. Use in a fume hood. |
|
| Company Name: |
Energy Chemical
|
| Tel: |
021-021-58432009 400-005-6266 |
| Website: |
http://www.energy-chemical.com |
| Company Name: |
Sigma-Aldrich
|
| Tel: |
021-61415566 800-8193336 |
| Website: |
https://www.sigmaaldrich.cn |
|