| Identification | Back Directory | [Name]
Diazomethane | [CAS]
334-88-3 | [Synonyms]
DIAZIRINE
azimethane
azimethylene
Diazomethane
Methane, diazo-(8CI,9CI) | [EINECS(EC#)]
206-382-7 | [Molecular Formula]
CH2N2 | [MOL File]
334-88-3.mol | [Molecular Weight]
42.04 |
| Chemical Properties | Back Directory | [Appearance]
Diazomethane is a flammable, yellow gas or
a liquid under pressure. Musty odor. | [Melting point ]
-145° | [Boiling point ]
bp -23° | [density ]
1.45 g/cm3 | [refractive index ]
1.4180 (estimate) | [form ]
Yellow gas | [Odor]
Musty odor (no accepted threshold value) | [Exposure limits]
TLV-TWA 0.2 ppm (0.38 mg/m3 ) (ACGIH)
PEL-TWA 0.2 ppm (0.38 mg/m3 ) (OSHA). | [IARC]
3 (Vol. 7, Sup 7) 1987 | [EPA Substance Registry System]
Diazomethane (334-88-3) |
| Hazard Information | Back Directory | [Definition]
ChEBI: The simplest diazo compound, in which a diazo group is attached to a methylene group. | [General Description]
Yellow gas with a musty odor. Highly toxic by inhalation Shipped as a liquid under pressure. | [Air & Water Reactions]
Reacts with water, releasing nitrogen, more stable in ether or dioxane. | [Reactivity Profile]
Diazomethane undergoes violent thermal decomposition. Above 200°C. the vapors may explode violently if rough glass surfaces are present. Explosions at low temperatures can occur if traces of organic matter are present. [J. Phys. Chem. 35:1403(1931)]. Produces explosions with alkali metals. Reacts with copper powder and to some extent all solid surfaces to produce nitrogen and solid white polymethylene. Reacts with dimethylaminodimethylarsine and trimethyltin in ether with vigorous foaming. | [Potential Exposure]
Diazomethane is a powerful methylat-
ing agent for acidic compounds, such as carboxylic acids,
phenols and enols. It is used in pesticide manufacture and
pharmaceutical manufacture. | [First aid]
If this chemical gets into the eyes, remove any
contact lenses at once and irrigate immediately for at least
15 minutes, occasionally lifting upper and lower lids. Seek
medical attention immediately. If this chemical contacts the
skin, remove contaminated clothing and wash immediately
with soap and water. Seek medical attention immediately.
If this chemical has been inhaled, remove from exposure,
begin rescue breathing (using universal precautions, includ-
ing resuscitation mask) if breathing has stopped and CPR if
heart action has stopped. Transfer promptly to a medical
facility. When this chemical has been swallowed, get medi-
cal attention. Give large quantities of water and induce
vomiting. Do not make an unconscious person vomit.
Medical observation is recommended for 24 to 48 hours
after breathing overexposure, as pulmonary edema may be
delayed. As first aid for pulmonary edema, a doctor or
authorized paramedic may consider administering a drug or
other inhalation therapy. If frostbite has occurred, seek
medical attention immediately; do NOT rub the affected
areas or flush them with water. In order to prevent further
tissue damage, do NOT attempt to remove frozen clothing
from frostbitten areas. If frostbite has NOT occurred, imme-
diately and thoroughly wash contaminated skin with soap
and water. | [Shipping]
UN1953 Compressed gas, toxic, flammable, n.o.s. | [Incompatibilities]
Heat (at about or above 100
C), shock,
friction, concussion, sunlight, or other intense illuminations
may cause explosions. Contact with alkali metals; drying
agents such as calcium sulfate, or rough edges (such as
ground glass) may cause explosions. Diazo compounds can
detonate. This applies in particular to organic azides that
have been sensitized by the addition of metal salts or strong
acids. Toxic gases are formed by mixing materials of this
class with acids, aldehydes, amides, carbamates, cyanides,
inorganic fluorides, halogenated organics, isocyanates,
ketones, metals, nitrides, peroxides, phenols, epoxides, acyl
halides, and strong oxidizing or reducing agents.
Flammable gases are formed by mixing materials in this
group with alkali metals. Explosive combination can occur
with strong oxidizing agents, metal salts, peroxides, and
sulfides. This chemical is sensitive to prolonged exposure
to heat. This chemical is incompatible with strong
oxidizing agents
. | [Chemical Properties]
Diazomethane is a flammable, yellow gas or
a liquid under pressure. Musty odor. | [Waste Disposal]
Decompose chemically
with ceric ammonium nitrate under constant agitation and
cooling
. | [Production Methods]
Two main methods are used to prepare diazomethane. One uses commercially available apparatus specifically designed for its preparation and distillation while entrained with ether. The resulting ether solution is typically of 0.3–0.4 m concentration and diazomethane is in its purest form. Such apparatus have specialized joints without ground glass and come in a range of sizes for generating diazomethane on scales of around 1, 50, or 300 mmol. The other method uses conventional glassware. Both methods use hydroxide to generate the diazomethane from nitrosamide precursors. The more formal method involves adding N-methyl-N-nitroso-toluenesulfonamide (Fig. 1), also known as Diazald, to KOH. The manufacturer's instructions for the use of this apparatus should be followed explicitly.
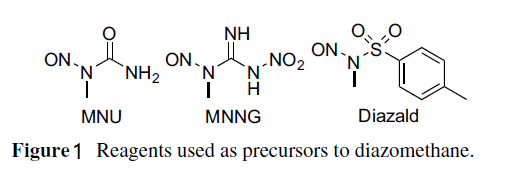
Figure 1 Reagents used as precursors to diazomethane.
The home brew method to make diazomethane can be found in its original form in Organic Syntheses (using N-methyl-N-nitrosourea as the precursor) (De Boer and Backer, 1956). This method uses a two-phase system of 50% aqueous KOH and diethyl ether in an Erlenmeyer flask cooled in an ice-water bath and stirred magnetically. The precursor recommended today, because it is safer to store and handle, is the crystalline solid N-methyl-N-nitroso-nitro-guanidine (MNNG). However, MNNG is still considered toxic, a severe irritant, a carcinogen, and a mutagen, and is typically used for generation of diazomethane quantities of 1 mmol. MNNG is slowly added to the two-phase system portion-wise. Sufficient precursor must be used to allow for materials transfer losses of diazomethane that are inevitable in the incomplete separation procedures described following. A yellow color will develop in the ether phase as the diazomethane is generated. After all of the precursor has been added, the solutions may be stirred for 10 min or so to allow the reaction to complete. The upper ether layer is decanted into a clean flask held in an ice-water bath. DO NOT use a separatory funnel with a ground-glass stopcock to separate the aqueous solution from the ether phase. Another portion of ether is added to the reaction flask, and it is stirred at ice-water bath temperature to extract remaining diazomethane. This ether layer is also decanted into the clean flask in the ice-water bath. This process may be repeated. The combined ether phases are likely to contain some dissolved water, which may be removed by adding KOH pellets and allowing the solution to stand in an ice-water bath for 0.5–3 h. The resulting yellow ethereal solution of diazomethane is ready for use. This procedure can be conducted on up to a 60 mmol scale. | [Health Hazard]
Diazomethane vapor causes severe irritation of the skin, eyes, mucous membranes,
and lungs. It is considered to be a substance with poor warning properties, and the
effects of exposure may be delayed in onset. Symptoms of exposure may include
headache, chest pain, cough, fever, severe asthmatic attacks, and pulmonary edema,
which can be fatal. Exposure of the skin and mucous membranes to diazomethane
may cause serious burns.
Diazomethane is a powerful allergen. Prolonged or repeated exposure to
diazomethane can lead to sensitization of the skin and lungs, in which case asthma-
like symptoms or fever may occur as the result of exposure to concentrations of
diazomethane that previously caused no symptoms. Chronic exposure to
diazomethane has been reported to cause cancer in experimental animals, but this
substance has not been identified as a human carcinogen.
Note that diazomethane is often prepared in situ from precursors that may
themselves be highly toxic and/or carcinogenic. | [Health Hazard]
It is a highly toxic gas and an irritant toeye, nose, and the entire respiratory tract.Exposure can cause dizziness, weakness,chest pain, severe headache, fever, asthmaticattack and pneumonia. Exposure to traceconcentrations of this substance can alsoproduce adverse effects, causing coughing,wheezing and headache. There have beenmany reported cases of poisoning. Its toxicitymay be attributed to its strong methylatingproperty. | [Fire Hazard]
Pure diazomethane gas and liquid are readily flammable and can explode easily. A
variety of conditions have been reported to cause explosions of diazomethane,
including contact with rough surfaces such as ground-glass joints, etched or
scratched flasks, and glass tubing that has not been carefully fire-polished. Direct
sunlight and strong artificial light may also cause explosions of this substance.
Violent reactions may occur on exposure of diazomethane to alkali metals. | [Flammability and Explosibility]
Pure diazomethane gas and liquid are readily flammable and can explode easily. A variety of conditions have been reported to cause explosions of diazomethane, including contact with rough surfaces such as ground-glass joints, etched or scratched flasks, and glass tubing that has not been carefully fire-polished. Direct sunlight and strong artificial light may also cause explosions of this substance. Violent reactions may occur on exposure of diazomethane to alkali metals. | [Synthesis]
To a solution of potassium hydroxide (30 mL,40%) in ether (100 ml), cooled below 5 °C with ice bath, was added in batches α-nitroso-α-methylurea with stirring. The organic phase was separated and dried over globosity potassium hydroxide for 3 hours. There is diazomethane (~2.6 g) in the ether solution, which was used without further purification. | [Carcinogenicity]
Diazomethane was administered
to rats and mice by inhalation, dermal, or subcutaneous
injection routes using concentrations of 0.1 or 3.3 mg/mL.
Mice developed lung tumors following either dermal application
or inhalation at both concentrations. | [storage]
diazomethane should preferably be handled in solution using glassware specially designated for diazomethane (e.g., with Clear-Seal joints) and should be used as soon as possible after preparation. Storage of diazomethane solutions (even at low temperature) is not advisable. All work with diazomethane should be conducted in a fume hood behind a safety shield, and appropriate impermeable gloves, protective clothing, and safety goggles should be worn at all times. |
| Questions And Answer | Back Directory | [Preparation]
Diazomethane was generated with a diazomethane-generating glassware kit (Aldrich). A solution of N-methyl-N-nitroso-4-toluenesulfonamide (Diazald, 2.23 g, 10.4 mmol) in ether (24 mL) was added dropwise to a mixture of KOH (1.75 g, 31.2 mmol) in H2O (18 mL), ether (4 mL), and 2-(2-ethoxyethoxy)ethanol (18 mL) kept at 70 oC. The ethereal solution of diazomethane was continuously distilled into a flask that was ready to use for the next reaction. | [Description]
At room temperature, diazomethane (CH2N2) is a toxic yellow gas which can cause significant irritation upon inhalation. Solutions that contain concentrated diazomethane are highly toxic, and they can result in explosions. When heated, Diazomethane emits toxic fumes made of nitrogen oxides; the toxic fumes are also independent of its decomposition. Diazomethane is used after its preparation in ether or in ether that contains traces of ethanol. It is rarely prepared and applied to other solvents which may include dichloromethane. Diazomethane is related to hydrogen cyanide and formaldehyde in its annotation.
| [Physical and Chemical Properties]
Diazomethane has a molecular weight of 42.041 g/mol, and a monoisotopic mass of 42.022 g/mol, which is also its exact mass. Diazomethane has a musty odor, and it is shipped in the form of compressed gas. It has a boiling point of -90 F or -230 C at 760 mm Hg and a melting point of -2290 F or -1450 C.
Diazomethane dissolves in water, and it is highly soluble in dioxane and ether. It has a relative density of 1.45 (water=1) and a relative vapor density 1.45 (air=1).
Copper powder influences the decomposition of diazomethane where nitrogen evolves, and polymethylene’s insoluble white flakes are formed.
| [Uses]
It is used as a methylating agent especially for acidic compounds which may include enols, phenols, and carboxylic acids.
| [Methods of Preparation]
Diazomethane can be manufactured from an alkali (potassium carbonate or potassium hydroxide) reaction With N-Nitrosomethyl-P-Toluenesulfonamide. It can also be prepared from ether solutions of diazomethane, where N-nitroso-beta-methylaminoisobutyl methyl ketone in isopropanol and ether is reacted with sodium isopropoxide.
Gaseous diazomethane is prepared from hydrazine and chloroform through a reaction with potassium hydroxide or from nitrosomethylurea and potassium hydroxide.
| [Precautions]
Inhalation, eye or skin contact with diazomethane results in eye irritation, shortness of breath, coughing, headache, chest pains, pulmonary edema and fever.
|
| Safety Data | Back Directory | [Hazard Codes ]
T | [Risk Statements ]
45 | [Safety Statements ]
53-45 | [RIDADR ]
1953 | [Autoignition Temperature]
150 °C; impure material explodes at lower temperature | [HazardClass ]
2.3 | [Safety Profile]
Confirmed carcinogen
with experimental tumorigenic data. A
poisonous irritant by inhalation. A powerful
allergen. It can cause pulmonary edema and
frequently causes hypersensitivity leading to
asthmatic symptoms. Mutation data
reported. Highly explosive when shocked,
exposed to heat, or by chemical reaction.
Undiluted liquid or gas may explode on
contact with alkali metals, rough surfaces,
heat (lOO°C), hgh-intensity light, or shock.
When heated to decomposition or on
contact with acid or acid fumes it emits
highly toxic fumes of NOx. Incompatible
with alkali metals; calcium sulfate. | [Hazardous Substances Data]
334-88-3(Hazardous Substances Data) | [Toxicity]
LCLO inhal (cat) 175 ppm (10 min)
PEL (OSHA) 0.2 ppm (0.4 mg/m3)
TLV-TWA (ACGIH) 0.2 ppm (0.4 mg/m3) | [IDLA]
2 ppm |
|
| Company Name: |
HBCChem, Inc.
|
| Tel: |
+1-510-219-6317 |
| Website: |
www.warehouse-sample-usa.com |
| Company Name: |
SKC Inc.
|
| Tel: |
724-941-9701 |
| Website: |
www.skcinc.com |
|