| Identification | Back Directory | [Name]
(S)-4-N-Boc-2-(hydroxymethyl)piperazine | [CAS]
314741-40-7 | [Synonyms]
H57160
(S)-4-N-Boc-2-hydroxyMeth...
(3S)-3-(Hydroxymethyl)piperazine
(S)-1-Boc-3-(hyroxyMethyl)piperazine
(S)-1-Boc-3-hydroxymethyl-piperazine
(S)-4-Boc-2-(Hydroxymethyl)piperazine
(3S)-1-Boc-3-(hydroxyMethyl)-piperazine
tert-butyl (S)-3-(hydroxymethyl)piperazine-1-carboxylate
(S)-tert-Butyl 3-(hydroxymethyl)piperazine-1-carboxylate
tert-butyl (3S)-3-(hydroxyMethyl)piperazine-1-carboxylate
1-Piperazinecarboxylic acid, 3-(hydroxymethyl)-, 1,1-dimethylethyl ester, (3S)- | [Molecular Formula]
C10H20N2O3 | [MDL Number]
MFCD05863889 | [MOL File]
314741-40-7.mol | [Molecular Weight]
216.277 |
| Chemical Properties | Back Directory | [Boiling point ]
322.9±22.0 °C(Predicted) | [density ]
1.085±0.06 g/cm3(Predicted) | [storage temp. ]
Keep in dark place,Sealed in dry,2-8°C | [pka]
14.97±0.10(Predicted) | [CAS DataBase Reference]
314741-40-7 |
| Hazard Information | Back Directory | [Definition]
(S)-1-Boc-3-hydroxymethyl-piperazine is also known as (S)-4-N-Boc-2-(hydroxymethyl)piperazine or (S)-tert-Butyl 3-(hydroxymethyl)piperazine-1-carboxylate.
| [Uses]
(S)-4-N-Boc-2-(hydroxymethyl)piperazine is a useful research chemical. | [Synthesis]
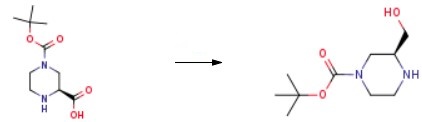
To synthesize (S)-1-Boc-3-hydroxymethyl-piperazine, a stirred suspension of (S)-piperazine-l,3-dicarboxylic acid l-tert-buty ester (5.00 g, 21.7 mmol) in THF (40 mL) was prepared. Slowly, 1.0 M borane-THF complex solution (32.6 mL, 32.6 mmol) was added to the suspension. The reaction mixture was heated to 90 ℃ and stirred under reflux for 2 h. Before further proceeding, the mixture was removed from heat and an additional 1.5 equivalents of 1.0 M borane-THF complex solution (32.6 mL, 32.6 mmol) was added. The reaction was then reheated and stirred under reflux for 2 h. Subsequently, the reaction mixture was cooled to 0 ℃ and quenched by slowly adding methanol. The resulting mixture was concentrated under reduced pressure, yielding a white solid. This solid was dissolved in THF (30 mL) and cooled to 0 ℃. Slowly, a 2.0 M solution of lithium aluminum hydride (LiAlH4) in THF (27 mL, 54.0 mmol) was added. The reaction was heated to 90 ℃ and stirred under reflux for 2 h. Another portion of 2.0 M LiAlH4 was added, and the reaction mixture was stirred under reflux for 4 hours, followed by overnight stirring at RT. The reaction mixture was then cooled to 0 ℃ and quenched by slowly adding 1.0 M aqueous sodium hydroxide (NaOH) solution until the exothermic reaction subsided. The product (S)-1-Boc-3-hydroxymethyl-piperazine was obtained after purification.
 |
|
|