| Identification | Back Directory | [Name]
DIETHYLPHOSPHONOACETIC ACID | [CAS]
3095-95-2 | [Synonyms]
NSC 272281
acetyl diethyl phosphate
(diethoxyphosphoryl)acetate
DIETHYLPHOSPHONOACETIC ACID
DIETHYLPHOSPHONOETHANOIC ACID
Diethoxyphosphorylacetic acid
DIETHYLCARBOXYMETHYLPHOSPHONATE
(Diethoxyphosphinyl)acetic acid
Diethylphosphonoacetic acid,98%
Diethylphosphonoacetic acid 95%
(DIETHOXYPHOSPHINOYL)ACETIC ACID
2-(Diethoxyphosphinyl)acetic acid
Diethyl carboxymethanephosphonate
2-(diethoxyphosphoryl)acetic acid
Acetic acid,2-(diethoxyphosphinyl)-
Diethylphosphonoacetic acid, 98% 25GR
Diethylcarboxymethylphosphonate, 98 %
Phosphono-acetic acid P,P-diethyl ester
Diethyl (hydroxycarbonylmethyl)phosphonate
(Diethoxyphosphoryl)acetic acid, Diethylphosphonoacetic acid | [EINECS(EC#)]
608-560-3 | [Molecular Formula]
C6H13O5P | [MDL Number]
MFCD00192032 | [MOL File]
3095-95-2.mol | [Molecular Weight]
196.14 |
| Chemical Properties | Back Directory | [Appearance]
clear colorless to yellow viscous liquid | [Boiling point ]
150 °C0.05 mm Hg(lit.)
| [density ]
1.220 g/mL at 25 °C(lit.)
| [refractive index ]
n20/D 1.445(lit.)
| [Fp ]
>230 °F
| [storage temp. ]
Store at 0-5°C | [form ]
Viscous Liquid | [pka]
3.48±0.10(Predicted) | [color ]
Clear colorless to yellow | [Specific Gravity]
1.220 | [InChI]
InChI=1S/C6H13O5P/c1-3-10-12(9,11-4-2)5-6(7)8/h3-5H2,1-2H3,(H,7,8) | [InChIKey]
DVQMPWOLBFKUMM-UHFFFAOYSA-N | [SMILES]
C(O)(=O)CP(OCC)(OCC)=O |
| Hazard Information | Back Directory | [Chemical Properties]
clear colorless to yellow viscous liquid | [Description]
Diethylphosphonoacetic Acid (DPA) is a Horner-Wadsworth-Emmons (HWE) reagent. The mixed anhydride of trifluoroacetic acid—diethylphosphonoacetic acid Treat a 21-hydroxy-20-keto steroid leads directly to cardenolides by an intramolecular Horner-Emmons reaction. It could also used as a dummy template to prepare a molecularly imprinted polymer for use as an artificial receptor for organophosphorus pesticides (OPPs)[1-3]. | [Uses]
Diethylphosphonoacetic Acid is a reagent that is used for enantioselective preparation of α-phosphoryl-α,β-unsatd.-δ-aryl-δ-lactones from nonracemic β-hydroxyaldehydes and their conversion to α-methylene-δ-aryl-δ-lactones. | [Production Methods]
Diethylphosphonoacetic acid is synthesised using triethyl phosphonoacetate as a raw material by chemical reaction. The specific synthesis steps are as follows:
After dissolving triethyl phosphonoacetate (1.7 g, 7.5 mmol) in ethanol / water (15 mL ie 14: 1) at room temperature, potassium hydroxide (424.2 mg, 7.56 mmol, ) 6597-4400) was added and stirred at room temperature. The reaction was neutralized with 1N HCl to pH 4, the ethanol was removed by distillation under reduced pressure, diluted with chloroform (20 mL), washed with water (10 mL) and saturated brine (10 mL). The separated organic layer was dried over anhydrous sodium sulfate, filtered and distilled under reduced pressure to obtain 2-(diethoxyphosphoryl)acetic acid 4 (1.48 gm, 100percent).
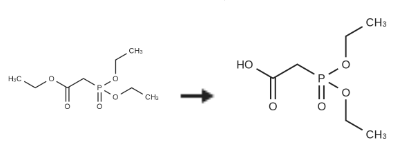 | [Synthesis Reference(s)]
Journal of the American Chemical Society, 102, p. 4534, 1980 DOI: 10.1021/ja00533a047 | [reaction suitability]
reaction type: C-C Bond Formation | [References]
[1] S. Donovan, J. Mcmurry, M. Avery. “Synthesis of digitoxigenin by remote functionalization.” Tetrahedron Letters 20 1 (1979): 3287–3290.
[2] A. M. Boldi, Hisham O. Eissa, Charles R. Johnson. “Solid-phase library synthesis of triazolopyridazines via [4+2] cycloadditions.” Tetrahedron Letters 40 1 (1999): 619–622.
[3] Lu Zhang. “Preparation and Characterization of Broad-Spectrum Artificial Antibody for OPPs Based on Dummy Template Imprinting Technique.” International Journal of Polymer Analysis and Characterization 117 1 (2014): 510–521. |
|
|