| Identification | Back Directory | [Name]
2-NAPHTHALENOL, 3-BROMO- | [CAS]
30478-88-7 | [Synonyms]
3-broMonaphthalen-2-ol
3-Bromo-2-naphthol 99%
2-NAPHTHALENOL, 3-BROMO- | [Molecular Formula]
C10H7BrO | [MDL Number]
MFCD10000951 | [MOL File]
30478-88-7.mol | [Molecular Weight]
223.07 |
| Chemical Properties | Back Directory | [Melting point ]
82 °C | [Boiling point ]
313.7±15.0 °C(Predicted) | [density ]
1.614±0.06 g/cm3(Predicted) | [storage temp. ]
Keep in dark place,Inert atmosphere,Room temperature | [form ]
powder to crystal | [pka]
8.03±0.40(Predicted) | [color ]
White to Light yellow | [CAS DataBase Reference]
30478-88-7 |
| Hazard Information | Back Directory | [Uses]
3-Bromo-2-naphthol is an important organic compound with a wide range of applications in the pharmaceutical and chemical industries. It is commonly used in organic synthetic materials to prepare pharmaceutical products, ester compounds, resin compositions, curing products and stacking films; it serves as an important reagent and catalyst in a variety of chemical reactions. | [Synthesis]
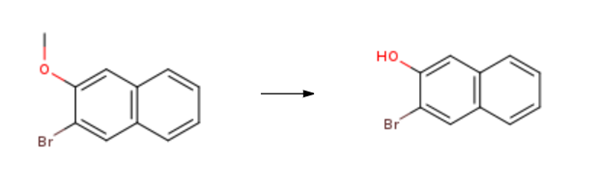
3-Bromo-2-naphthol is synthesised using 2-bromo-3-methoxynaphthalene as a raw material by chemical reaction. The specific synthesis steps are as follows:
General procedure: To a Schlenk flask were added 2-bromo-3-methoxynaphthalene(1.0 equiv), aryl boronic acid (2.2 equiv), K2CO3 (3.0 equiv), Pd(PPh3)4 (2.5 mol%), and degassed EtOH/toluene/water (1/1/1) under Ar atmosphere. The mixture was heated at 90 °C until thecompletion of the reaction. Then the mixture was cooled to room temperature, and DCM was added. The mixture was washed with NaOH solution (20% wt), and the aqueous phase was extracted with DCM (2 × 20 mL). The combined organic phase was washed with brine (20 mL) and dried overanhydrous MgSO4. After removing the solvent, the residue was dissolved in anhydrous DCM. Thesolution was cooled to -78 °C, and BBr3 (1 M in DCM, 5.0 equiv) was added slowly by syringe. Thenthe mixture was warmed up to room temperature and stirred until the complete consumption of thestarting material. The mixture was poured into the ice water (50 mL) and extracted with DCM (3 × 50mL). The combined organic phase was washed with brine (100 mL) and dried over anhydrous Na2SO4. After removing the solvent, the residue was purified by silica gel chromatography to give 3-Bromo-2-naphthol.
|
|
|