| Identification | Back Directory | [Name]
3,5-PYRAZOLEDICARBOXYLIC ACID MONOHYDRATE | [CAS]
303180-11-2 | [Synonyms]
TIMTEC-BB SBB003804
LABOTEST-BB LT00455359
3,5-PYRAZOLEDICARBOXYLIC ACID MONOHYDRATE
1H-Pyrazole-3,5-dicarboxylic acid, hydrate
PYRAZOLE-3,5-DICARBOXYLIC ACID MONOHYDRATE
3,5-Pyrazoledicarboxylic acid monohydrate 97%
1H-Pyrazole-3,5-dicarboxylic acid, hydrate (1:1)
1H-Pyrazole-3,5-dicarboxylic acid monohydrate, 98%
Pyrazole-3,5-dicarboxylic acid monohydrate for synthesis | [EINECS(EC#)]
221-474-7 | [Molecular Formula]
C5H6N2O5 | [MDL Number]
MFCD00149323 | [MOL File]
303180-11-2.mol | [Molecular Weight]
174.11 |
| Questions And Answer | Back Directory | [Form]
Solid | [Structure]
In the 3,5-Pyrazoledicarboxylic acid monohydrate, C5H4N2O4·H2O, the 3,5-pyrazoledicarboxylic acid (H3pdc) molecules are joined into one-dimensional chains by O-H···O and N-H···O hydrogen bonds, with distances of 2.671 (2) and 2.776 (2) A, respectively. The one-dimensional chains form a three-dimensional structure via O-H···OW and OW-HW···N hydrogen bonds, with distances of 2.597 (3) and 2.780 (3) A, respectively. In addition to the potential for forming open-channel frameworks, access to the six coordination atoms of H3pdc can be directly controlled by varying the pH of the reaction environment, allowing further control over the design and synthesis of novel coordination polymers using various metal centres.
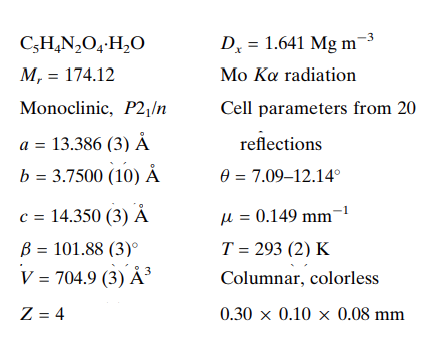 In addition to the potential for forming open-channel frameworks, access to the six coordination atoms of H3pdc can be directly controlled by varying the pH of the reaction environment, allowing further control over the design and synthesis of novel coordination polymers using various metal centres. H3pdc possesses three different protonated hydrogens: Ha, Hb, and Hc. Although both Ha and Hb are attached to carboxylic oxygen atoms, the two experience quite different coordination environments. Hc in this ligand is linked to a nitrogen atom and is more difficult to deprotonate than the other two hydrogen atoms. The difference in the binding power of these protons allows their deprotonation at different pH levels. The flexible, multifunctional coordination sites of this ligand also give a high likelihood for generating coordination polymers with high dimensions[1-2]. In addition to the potential for forming open-channel frameworks, access to the six coordination atoms of H3pdc can be directly controlled by varying the pH of the reaction environment, allowing further control over the design and synthesis of novel coordination polymers using various metal centres. H3pdc possesses three different protonated hydrogens: Ha, Hb, and Hc. Although both Ha and Hb are attached to carboxylic oxygen atoms, the two experience quite different coordination environments. Hc in this ligand is linked to a nitrogen atom and is more difficult to deprotonate than the other two hydrogen atoms. The difference in the binding power of these protons allows their deprotonation at different pH levels. The flexible, multifunctional coordination sites of this ligand also give a high likelihood for generating coordination polymers with high dimensions[1-2].
|
| Chemical Properties | Back Directory | [Melting point ]
292-295 °C (dec.)(lit.)
| [storage temp. ]
Store below +30°C. | [form ]
powder to crystal | [color ]
White to Almost white | [BRN ]
147821 | [InChI]
InChI=1S/C5H4N2O4.H2O/c8-4(9)2-1-3(5(10)11)7-6-2;/h1H,(H,6,7)(H,8,9)(H,10,11);1H2 | [InChIKey]
GLINCONFUZIMCN-UHFFFAOYSA-N | [SMILES]
C1(C(=O)O)=NNC(C(=O)O)=C1.O |
| Hazard Information | Back Directory | [Uses]
3,5-PYRAZOLEDICARBOXYLIC ACID MONOHYDRATE is a useful research chemical. | [References]
[1] Ching. “3,5-Pyrazoledicarboxylic acid monohydrate.” Acta crystallographica. Section C, Crystal structure communications 56 (Pt 9) (2000): 1124–5.
[2] Irena Kostova, Wolfgang Kiefer, Niculina Peica. “Raman, FT-IR, and DFT studies of 3,5-pyrazoledicarboxylic acid and its Ce(III) and Nd(III) complexes.” Journal of Raman Spectroscopy 38 1 (2006): 1–10.
|
|
|