| Identification | Back Directory | [Name]
DIBROM | [CAS]
300-76-5 | [Synonyms]
BRP
NALED
oms75
Alvora
DIBROM
BROMEX
hibrom
naledu
fosbrom
RE-4355
Trumpet
bromex50
ent24988
flibolex
nikabrom
ENT 24988
Dibromfos
Bromex 50
ortho4355
Dibrom(R)
orthodibrom
o-Dibrom 8E
arthodibrom
BROMCHLOPHOS
Ortho-Dibrom
orthodibromo
BRP Standard
bromochlorphos
DIBROM (NALED)
Naled dibromo-
naled,[liquid]
Naled E.C.(50%)
Ortho Dibrom 8E
DIBROM, 250MG, NEAT
bromex(insecticide)
Naled Solution, 100ppm
Naled 250mg [300-76-5]
naled (bsi,iso,ansi,esa)
DIBROM PESTANAL (1,2-DIBROMO-2,2-DI CHLO
o,2,2-dichloro-1,2-dibromoethylphosphate
dimethyl1,2-dibromo-2,2-dichloroethylphosphate
DIMETHYL-1,2-DIBROMO-2-DICHLOROETHYL-PHOSPHATE
dimethyl-1,2-dibromo-2,2-dichloroethylphosphate
Dimethyl 1,2-dibromo-2,2-dichloroethyl phosphate
1,2-dibromo-2,2-dichloro-ethanodimethylphosphate
1,2-DIBROMO-2,2-DICHLOROETHYL DIMETHYL-PHOSPHATE
1,2-DIBROMO-2,2-DICHLOROETHYLDIMETHYLESTERPHOSPHATE
o,o-dimethylo-2,2-dichloro-1,2-dibromoethylphosphate
o-(1,2-dibromo-2,2-dicloro-etil)-o,o-dimetil-fosfato
O,O'-DIMETHYL-1,2-DIBROMO-2,2-DICHLOROETHYL PHOSPHATE
1,2-Dibromo-2,2-dichloroethyl dimethyl phosphatic acid
O-(1,2-dibromo-2,2-dichloethyl )-0,0-dimethylphosphate
Phosphoric acid 1,2-dibromo-2,2-dichloroethyl dimethyl
o-(1,2-dibroom-2,2-dichloor-ethyl)-o,o-dimethyl-fosfaat
o-(1,2-dibrom-2,2-dichlor-aethyl)-o,o-dimethyl-phosphat
o,o-dimethyl-o-(1,2-dibrom-2,2-dichlor-aethyl)-phosphat
o,o-dimethyl-o-(1,2-dibromo-2,2-dichloroethyl)phosphate
fosforano-1,2-dwubromo-2,2-dwuchloroetylo-o,o-dwumetylowy
phosphoricacid,1,2-dibromo-2,2-dichloroethyldimethylester
naled (ISO) 1,2-dibromo-2,2-dichloroethyl dimethyl phosphate
Phosphoric acid, 1,2-dibromo-2,2-dichloroethyl dimethyl ester
phosphatedeo,o-dimethyleetdeo-(1,2-dibromo-2,2-dichlorethyle)
phosphatedeo,o-dimethyleetdeo-(1,2-dibromo-2,2-dichlorethyle)(french)
(1,2-Dibromo-2,2-dichloroethyl) dimethyl phosphate, Bromchlophos, Naled | [EINECS(EC#)]
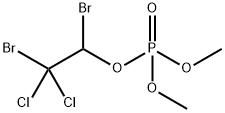
206-098-3 | [Molecular Formula]
C4H7Br2Cl2O4P | [MDL Number]
MFCD00053227 | [MOL File]
300-76-5.mol | [Molecular Weight]
380.78 |
| Chemical Properties | Back Directory | [Appearance]
Naled is a white crystalline solid (when pure)
or light straw-colored liquid (above 26.7℃) with a slightly
pungent insecticide odor. | [Melting point ]
212℃ (decomposition) | [Boiling point ]
110℃ (0.5 Torr) | [density ]
1.96 g/cm3 | [vapor pressure ]
2 (quoted, Verschueren, 1983) | [refractive index ]
1.5108 (28℃) | [storage temp. ]
0-6°C
| [solubility ]
Freely soluble in ketone, alcohols, aromatic and chlorinated hydrocarbons but sparingly soluble in
petroleum solvents and mineral oils (Windholz et al., 1983) | [form ]
solid | [Water Solubility ]
2000mg l-1(20 °C) | [Specific Gravity]
1.96 (20℃) | [Merck ]
13,6384 | [BRN ]
2049930 | [Exposure limits]
NIOSH REL: TWA 3 mg/m3, IDLH 200 mg/m3; OSHA PEL: TWA 3
mg/m3; ACGIH TLV: TWA 3 mg/m3. | [Stability:]
Light Sensitive, Moisture Sensitive | [Contact allergens]
Naled is an organophosphate cholinesterase inhibitor
that is used as an insecticide and acaricide. Sensitization
seems to be very rare. | [EPA Substance Registry System]
Naled (300-76-5) |
| Hazard Information | Back Directory | [Chemical Properties]
Clear Colorless Liquid | [Uses]
A cholinesterase inhibitor. Insecticide; acaricide. | [Definition]
ChEBI: An dialkyl phosphate resulting from the formal condensation of the acidic hydroxy group of dimethyl hydrogen phosphate with the alcoholic hydroxy group of 1,2-dibromo-2,2-dichloroethanol. An organophosphate insecticide, it is no longer approved for use wit
in the European Union. | [Uses]
Insecticide; acaricide. | [General Description]
DIBROM is a white solid that may be dissolved in a liquid organic carrier with a pungent odor. DIBROM is a water emulsifiable liquid. DIBROM is insoluble in water and sinks in water. DIBROM can cause illness by inhalation, skin absorption and/or ingestion. The primary hazard is the threat to the environment. Immediate steps should be taken to limit its spread to the environment. If DIBROM is in liquid form, DIBROM can easily penetrate the soil and contaminate groundwater and nearby streams. DIBROM is used as a pesticide. | [Air & Water Reactions]
Practically insoluble in water [Farm Chemicals Handbook]. Hydrolyzed slowly in presence of water. | [Reactivity Profile]
DIBROM is incompatible with the following: Strong oxidizers, acids, sunlight, water [Note: Corrosive to metals. Hydrolyzed in presence of water.] . Unstable in presence of Iron [USCG, 1999]. Organophosphates are susceptible to formation of highly toxic and flammable phosphine gas in the presence of strong reducing agents such as hydrides. Partial oxidation by oxidizing agents may result in the release of toxic phosphorus oxides. | [Health Hazard]
INHALATION OR INGESTION: Symptoms secondary to cholinesterase inhibition are: headache, giddiness, nervousness, blurred vision, weakness, nausea, cramps, diarrhea, chest discomfort, sweating, miosis, tearing, salivation, and other excessive respiratory tract secretion, vomiting, cyanosis, muscle twitching, and convulsions. EYES: Irritating. SKIN: Irritating-can cause dermatitis. | [Fire Hazard]
May be combustible. (NOAA, 2007) | [Hazard]
Technical compound is a moderately volatile liquid. Bp 110C (0.5 mm Hg). Insoluble in water;
slightly soluble in aliphatic solvents; very soluble
in aromatic solvents; hydrolyzes in water. | [Potential Exposure]
A potential danger to those involved
in the manufacture, formulation, and application of this
insecticide, fungicide, bactericide, acaricide. Also used in
cooling towers, veterinary medicine, pulp and paper mill
systems; hospitals, swimming pools; and bathrooms. | [First aid]
If this chemical gets into the eyes, remove any
contact lenses at once and irrigate immediately for at least
15 minutes, occasionally lifting upper and lower lids. Seek
medical attention immediately. If this chemical contacts the
skin, remove contaminated clothing and wash immediately
with soap and water. Speed in removing material from skin
is of extreme importance. Shampoo hair promptly if contaminated.
Seek medical attention immediately. If this
chemical has been inhaled, remove from exposure, begin
rescue breathing (using universal precautions, including
resuscitation mask) if breathing has stopped and CPR if
heart action has stopped. Transfer promptly to a medical
facility. When this chemical has been swallowed, get medical
attention. Give large quantities of water and induce
vomiting. Do not make an unconscious person vomit.
Effects may be delayed. Medical observation is recommended.
| [Shipping]
UN2811 Toxic solids, organic, n.o.s., Hazard
Class: 6.1; Labels: 6.1-Poisonous materials, Technical
Name Required. UN2783 Organophosphorus pesticides,
solid, toxic, Hazard Class: 6.1; Labels: 6.1-Poisonous materials.
UN3018 Organophosphorus pesticides, liquid, toxic,
Hazard Class: 6.1; Labels: 6.1-Poisonous materials. | [Incompatibilities]
Incompatible with oxidizers (chlorates,
nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions.
Keep away from alkaline materials, strong bases,
strong acids, oxoacids, epoxides. Hydrolyzed in presence of
water. Degraded by sunlight. Decomposes when heated; on
contact with acids, acid fumes; bases, producing fumes of
hydrogen chloride, hydrogen bromide, phosphorous oxides.
Reacts with acids, strong oxidizers in sunlight. Slowly
reacts with water; hydrolysis is; corrosive to metals.
Attacks some plastics, rubber and coatings. | [Description]
Naled is an organophosphate insecticide and acaricide that inhibits acetylcholinesterase (AChE) and butyrylcholinesterase (BChE).1 It reduces the number of A. sollicitans mosquitos by 97% after one hour when aerially applied at a concentration of 0.1 pounds per acre.2 Naled (125 ppm AI) induces 100 and 64% mortality of T. telarius adults and immature mites, respectively, in an immediate contact toxicity test but does not induce mortality in mite eggs.3 It is toxic to rats with an LD50 value of 250 mg/kg.4 Formulations containing naled have been used in the control of mosquitoes in public areas and of crop-damaging insects in agriculture. | [Description]
Sensitization to Naled seems to be rare. | [Waste Disposal]
This pesticide is more
stable to hydrolysis than dichlorvos (50% hydrolysis at pH
9 @ 37.5℃ in 301 minutes). It is unstable in alkaline conditions,
in presence of iron; and is degraded by sunlight.
About 10% hydrolysis per day is obtained in ambient
water. Incineration is recommended for large amounts.
In accordance with 40CFR165, follow recommendations for the disposal of pesticides and pesticide containers. Must
be disposed properly by following package label directions
or by contacting your local or federal environmental control
agency, or by contacting your regional EPA office. | [Physical properties]
Colorless to pale yellow liquid or solid with a pungent odor | [Agricultural Uses]
Insecticide, Fungicide, Bactericide, Acaricide: Naled is a fast-acting, nonsystemic contact and stomach
insecticide used to control aphids, mites, mosquitoes,and flies on crops and in greenhouses, mushroom houses,
animal and poultry houses, kennels, food-processing
plants, and aquaria and in outdoor mosquito control. Liquid
formulations can be applied to greenhouse heating pipes to
kill insects by vapor action. It has been used by veterinarians
to kill parasitic worms (other than tapeworms) in dogs.
Naled may no longer be used in and around the home by
residents or professional applicators. Naled is available in
dust, emulsion concentrate, liquid, and ULV formulations.
Also used in cooling towers, veterinary medicine, pulp and
paper mill systems, hospitals, swimming pools, and bathrooms.
A U.S. EPA restricted Use Pesticide (RUP). Not
approved for use in EU countries. Registered for use in
the U.S. | [Trade name]
AI3-24988®; ARTHODIBROM®;
BROMCHLOPHOS®; BROMEX®; DIBROM®;
FLYKILLER®; LUCANAL®; HIBROM®; ORTHO®
4355; ORTHODIBROM®; ORTHODIBROMO®;
PROKIL® Naled; TRUMPET® | [Carcinogenicity]
When dogs were given 0.2, 2.0,
or 20 mg/kg/day naled by gavage for 1 year, cholinergic signs
(soft stools/diarrhea, salivation, and emesis), increases in
mineralization of spinal cord, and mild testicular degeneration
in males occurred at 2 and 20 mg/kg/day dose
levels . Erythrocyte and brain cholinesterase activities
were depressed at the same dose levels. Anemia also
occurred at 2 and 20 mg/kg/day, and erythrocyte count,
hemoglobin, and hematocrit were reduced. At 20 mg/kg/
day dose level, liver and kidney weights increased but
these were not accompanied by histopathological changes.
There was no evidence of carcinogenicity. | [Environmental Fate]
Chemical/Physical. Completely hydrolyzed in water within 2 days (Windholz et al.,
1983). In the presence of metals or reducing agents, naled loses bromine, forming dichlorvos
(Hartley and Kidd, 1987)
Naled emits toxic fumes of bromines, chlorides and phosphorus oxides when heated
to decomposition (Lewis, 1990). | [Metabolic pathway]
Naled is produced by the photochemical bromination of the dichlorovinyl
moiety of dichlorvos. The main route of naled metabolism and transformation
in the environment is through debromination to dichlorvos
which is probably the active cholinesterase inhibitor in vivo. Naled is also
rapidly hydrolysed to bromodichloroacetaldehyde in aqueous environments:
consequently much of its detoxification is likely to be via a nonenzymatic
hydrolytic route. | [Solubility in water]
Freely soluble in ketone, alcohols, aromatic and chlorinated hydrocarbons but sparingly soluble in
petroleum solvents and mineral oils (Windholz et al., 1983) | [Degradation]
Naled is stable when dry but it is rapidly hydrolysed in aqueous media
and more rapidly in alkaline and acidic media. It is degraded by sunlight.
In the presence of metals and reducing agents, bromine is lost and dichlorvos
(2) is formed (PM). In unbuffered aqueous solution naled was rapidly
hydrolysed via two routes, one leading to the formation of dimethyl
phosphate (3) and bromodichloroacetaldehyde (4) and the second via
desmethyhaled (5), which further decomposed to yield bromodichloroacetaldehyde
(4) and monomethyl phosphate (6) as shown in Scheme 1.
Naled was found to be much less hydrolytically stable than dichlorvos
and exhibited a maximum stability at pH 4 (Jentzen and Fischer, 1978). |
|
|