| Identification | Back Directory | [Name]
SILYDIANIN | [CAS]
29782-68-1 | [Synonyms]
SILYDIANIN
SILIDIANIN
SILYDIANIN(P)
Silydianin,SD
SILYDIANIN hplc
Silydianin (20 mg)
SILYDIANIN USP/EP/BP
Silydianin, 98%, from Silybum marianum (L.) Gaertn.
(3R,3aR,6R,7aR,8R)-7a-Hydroxy-8-(4-hydroxy-3-methoxyphenyl)-4-((2R,3R)-3,5,7-trihydroxy-4-oxochroman-2-yl)-3,3a,6,7a-tetrahydro-3,6-methanobenzofuran-7(2H)-one
(3R,8R)-2,3,3aβ,7a-Tetrahydro-7aβ-hydroxy-8-(4-hydroxy-3-methoxyphenyl)-4-[(2R,3R)-3,5,7-trihydroxy-3,4-dihydro-4-oxo-2H-1-benzopyran-2-yl]-3α,6α-methanobenzofuran-7(6H)-one
3,6-Methanobenzofuran-7(6H)-one,4-[(2R,3R)-3,4-dihydro-3,5,7-trihydroxy-4-oxo-2H-1-benzopyran-2-yl]-2,3,3a,7a-tetrahydro-7a-hydroxy-8-(4-hydroxy-3-methoxyphenyl)-,(3R,3aR,6R,7aR,8R)- | [EINECS(EC#)]
249-848-5 | [Molecular Formula]
C25H22O10 | [MDL Number]
MFCD00238684 | [MOL File]
29782-68-1.mol | [Molecular Weight]
482.44 |
| Chemical Properties | Back Directory | [Melting point ]
191℃ | [Boiling point ]
819.0±65.0 °C(Predicted) | [density ]
1.675±0.06 g/cm3(Predicted) | [storage temp. ]
2-8°C | [solubility ]
DMSO (Slightly), Methanol (Slightly, Sonicated) | [form ]
neat | [pka]
7.41±0.60(Predicted) | [color ]
White to Off-White | [Water Solubility ]
practically insoluble in water |
| Questions And Answer | Back Directory | [Structure]
The structure of silydianin is unique among flavonolignans and somewhat complicated, but it is biosynthesized analogously to other silymarin flavonolignans - by the radical coupling of taxifolin and coniferyl alcohol. The only substantial difference is in the radical coupling forming two new CeC bonds, creating a bicyclo structure and the fate of the primary alcoholic group of the coniferyl alcohol. In silydianin, intramolecular hemiacetalization occurs; thus, the unusual geminal ketone-hemiacetal structure is formed.
The structure of silydianin was initially resolved using X-ray diffraction data. Since the resolution of the original data was low, the misplacement of some atoms or bonds was possible. Silydianin was crystallized from MeOH, and the crystal was used for X-ray crystallography. The crystallographic data presented here agree with the previously published structure but in a significantly higher resolution (original data d = 1.64 ?, this paper’s data d = 0.82 ?).
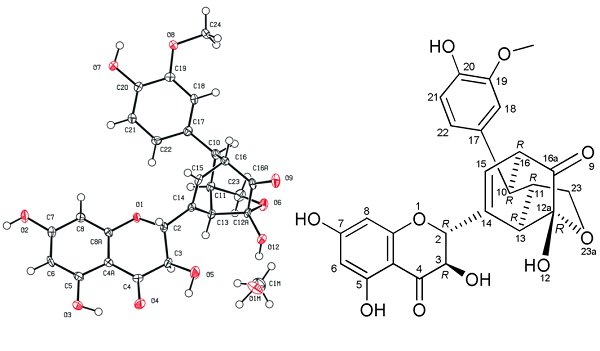 |
| Hazard Information | Back Directory | [Description]
Silydianin is a flavonolignan found in S. marianum and has diverse biological activities. It inhibits tyrosinase (IC50 = 2.6 μM for the mushroom enzyme) and scavenges DPPH radicals in a cell-free assay (IC50 = 66.8 μM). Silydianin (100 μM) protects against cytotoxicity induced by allyl alcohol, carbon tetrachloride, paracetamol (acetaminophen), or D-galactosamine in primary human hepatocytes. It inhibits the proliferation of DU145 and PC3 prostate cancer cells when used at a concentration of 90 μM. Silydianin (1%) prevents methacholine-induced airway hyperresponsiveness (AHR) and reduces blood and bronchoalveolar lavage fluid (BALF) levels of eosinophils in an ovalbumin-sensitized mouse model of allergic asthma when administered via inhalation.
| [Originator]
Silydianin was isolated for the first time by G. M?schlin (Dissertation thesis, Karlsruhe University, Germany, 1959) during initial experiments with S. marianum fruits as a compound named E5.
| [Uses]
Silydianin is an active constituent of Silybium marianum and is known the exhibit anti-inflammatory activity which regulates caspase-3 activation which affects cell membranes and acts as a free radica
l scavenger. | [Cytotoxicity]
Cell suspensions were incubated with different concentrations of silydianin (up to a final concentration of 1–100 μM) for 30 min at 37 °C in a dark incubator and untreated control samples. The samples were then centrifuged at 800–900 rpm, PMNs were resuspended in RPMI 1640, and 0.4% trypan blue and the viable and dead cells were scored. The cell viability was 94.8 ± 3.8% for silydianin at concentrations of 1–100 μM, indicating no cytotoxicity toward neutrophils[1].
| [References]
[1] David Biedermann . “Oxidation of flavonolignan silydianin to unexpected lactone-acid derivative.” Phytochemistry Letters 30 (2019): Pages 14-20.
[2] Ma?gorzata Zielińska-Przyjemska, Krzysztof Wiktorowicz. “An in vitro study of the protective effect of the flavonoid silydianin against reactive oxygen species.” Phytotherapy Research 20 2 (2006): 115–9.
|
|
|