| Identification | Back Directory | [Name]
N-(2-HYDROXYETHYL)-N-METHYL-4-TOLUIDINE | [CAS]
2842-44-6 | [Synonyms]
FirstCure MHPT
2-(N-Methyl-p-toluidino)
Hydroxyethyl methyltolylamine
2-(Methyl(p-tolyl)aMino)ethanol
2-(N-methyl-p-toluidino)ethanol
N-methyl-hydroxyethyl-p-toluidine
2-(N-Methyl-N-4-tolylamino)ethanol
2-(N-methyl-N-p-tolylamino)ethanol
N-Methyl-N-hydroxyethyl-P-toluidine
2-[Methyl(4-methylphenyl)amino]ethanol
N-(2-HYDROXYETHYL)-N-METHYL-4-TOLUIDINE
N-(2-Hydroxyethyl)-N-methyl-p-toluidine
N-Methyl-N-(2-hydroxyethyl)-p-toluidine
Ethanol, 2-[methyl(4-methylphenyl)amino]-
N-(2-HYDROXYETHYL)-N-METHYL-4-TOLUIDINE(MHPT)
N-(2-HYDROXYETHYL)-N-METHYL-PARA-TOLUIDINE(MPHT)
(5S,8R,9S,10S,13S,14S,17R)-17-ethynyl-3,3-difluoro-10,13-dimethyl-2,4,5,6,7,8,9,11,12,14,15,16-dodecahydro-1H-cyclopenta[a]phenanthren-17-ol | [EINECS(EC#)]
220-638-5 | [Molecular Formula]
C10H15NO | [MDL Number]
MFCD05663421 | [MOL File]
2842-44-6.mol | [Molecular Weight]
165.23 |
| Hazard Information | Back Directory | [Uses]
N-(2-HYDROXYETHYL)-N-METHYL-4-TOLUIDINE is an amine organic compound, which can be used as intermediate in organic synthesis. | [Synthesis]
Add reactant (0.2 mmol, 1.0 equiv), THF (2.0 mL) and CH2Br2 (0.6 mmol, 3.0 equiv) to a flame-dried 10 mL Schlenk tube in a glove box. Seal and take out of the glove box. Cool the reaction mixture to -78°C. Add nBuLi (0.56 mmol, 2.8 equiv) dropwise under N2 atmosphere within 3minutes. Stir the reaction at -78°C for 30 minutes and add ZnCl2 (0.1 mL, 0.5equiv, 1.0 M in Et2O). Allow the mixture to warm to room temperature and stir for 1 hour. Cool the mixture to 0°C. Add a premixture of H2O2 (30% in H2O, 0.5 mL) and NaOH (2.0 M, 1.0 mL). Stir the mixture at room temperature for another 1 hour and dilute with water (20 mL). Extract with DCM (30 mL x 2) and dry over Na2SO4. Filter and concentrate under vacuum. Purify the crude product by silica gel flash column chromatography to obtain product. 1H NMR (CDCl3, 500 MHz) δ 7.08 (d, J = 8.4 Hz, 2H), 6.78 (d, J = 8.4 Hz,2H), 3.80 (t, J = 5.6 Hz, 2H), 3.43 (t, J = 5.4 Hz, 2H), 2.93 (s, 3H), 2.28 (s, 3H), 2.01 (brs, 1H). 13C NMR (CDCl3, 125 MHz) δ 148.3, 129.8, 127.1, 114.0, 60.1, 56.2, 39.1, 20.4.
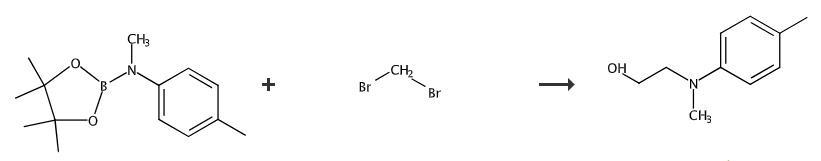 Fig The synthetic method of N-(2-hydroxyethyl)-N-methyl-4-toluidine
Fig The synthetic method of N-(2-hydroxyethyl)-N-methyl-4-toluidine
|
|
|