| Identification | Back Directory | [Name]
NIGERICIN SODIUM SALT | [CAS]
28380-24-7 | [Synonyms]
x-464
Helix c
HELIXIN C
NIGERICIN
Aids003767
Aids-003767
AZALOMYCIN M
NIGERICIN NA
POLYETHERIN A
Antibiotic M 2
ANTIBIOTIC K178
ANTIBIOTIC X-464
nigericin sodium
NIGERICIN, NA SALT
NIGERICIN APPOX. 95%
28643-80-3 (Sodium salt)
ANTIBIOTIC K178 SODIUM SALT
Nigericin from Streptomyces hygroscopicus
Antibiotic K178 sodium salt, Azalomycin M
nigericin sodium from streptomyces*hygroscopicus
NIGERICIN, SODIUM SALT, STREPTOMYCES HYGROSCOPICUS
Polyetherin A, AzaloMycin M, Helixin C, K 178, X 464
nigericin sodium salt from streptomyces hygroscopicus
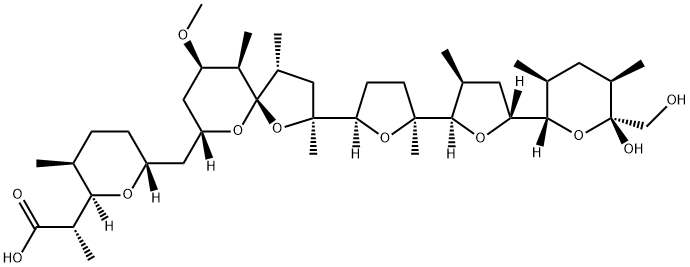
(2R)-2-((3S)-6-{[2-((5S)-5-{(3S)-5-[(3S,5R,6R)-6-Hydroxy-6-(hydroxymethyl)-3,5-dimethylperhydro-2H-pyran-2-yl]-3-methyloxolan-2-yl}-5-methyloxolan-2-yl)(2S,4R,9R,10R)-9-methoxy-2,4,10-trimethyl-1,6-dioxaspiro[4.5]dec-7-yl]methyl}-3-methylperhydro-2H-pyran-2-yl)propanoic acid | [Molecular Formula]
C40H67NaO11 | [MDL Number]
MFCD00036825 | [MOL File]
28380-24-7.mol | [Molecular Weight]
746.94 |
| Chemical Properties | Back Directory | [Melting point ]
183.5-185° | [alpha ]
D24 +36.2° (c = 0.842 in CHCl3) | [Boiling point ]
779.9±60.0 °C(Predicted) | [density ]
1.19±0.1 g/cm3(Predicted) | [storage temp. ]
2-8°C
| [solubility ]
chloroform: 10 mg/mL, clear, colorless
| [pka]
4.39±0.10(Predicted) | [InChIKey]
DANUORFCFTYTSZ-ANZYULKNSA-N |
| Safety Data | Back Directory | [Hazard Codes ]
T,Xi | [Risk Statements ]
25-36/37/38 | [Safety Statements ]
26-36/37/39-45-36 | [RIDADR ]
UN 3462 6.1/PG 3
| [WGK Germany ]
3
| [RTECS ]
QT6840000
| [F ]
10 | [HazardClass ]
6.1(b) | [PackingGroup ]
III | [HS Code ]
29419090 | [Toxicity]
LD50 in mice (mg/kg): 10-15 i.p. (Shoji); also reported as 2.5 i.p. (Harned) |
| Hazard Information | Back Directory | [Uses]
Nigericin is a polyether antibiotic produced by Streptomyces, notably S. hygroscopicus, isolated in the 1950s. Its complex structure was finally elucidated in 1968. Nigericin is an ionophore, possessing very high affinity for monovalent cations such as Na+ and K+. Nigericin disrupts membrane potential and Golgi apparatus in mitochondria. Although nigericin can be isolated as the free acid (under acidic conditions), like most ionophores it is extracted into organic solvents and is most conveniently isolated as a salt. In vitro, nigericin has broad biological activity against Gram positive bacteria, fungi, tumor cell lines and some viruses, including HIV. Nigericin is the most common member of the polyether class which are common false positives in in vitro screening bioassays using crude microbial extracts. They are thus important standards for dereplication. | [Uses]
Nigericin is a polyether antibiotic produced by Streptomyces, notably S. hygroscopicus, isolated in the 1950s. Its complex structure was finally elucidated in 1968. Nigericin is an ionophore, possessing very high affinity for monovalent cations such as Na+ and K+. Nigericin disrupts membrane potential and Golgi apparatus in mitochondria. Although nigericin can be isolated as the free acid (under acidic conditions), like most ionophores it is extracted into organic solvents and is most conveniently isolated as a salt. In vitro, nigericin has broad biological activity against Gram positive bacteria, fungi, tumour cell lines and some viruses, including HIV. Nigericin is the most common member of the polyether class which are common false positives in in vitro screening bioassays using crude microbial extracts. They are thus important standards for dereplication. | [Definition]
ChEBI: A polyether antibiotic which affects ion transport and ATPase activity in mitochondria. It is produced by Streptomyces hygroscopicus. |
|
|