| Identification | Back Directory | [Name]
1-Chloro-N,N,2-trimethylpropenylamine | [CAS]
26189-59-3 | [Synonyms]
Ghosez′s reagent
TETRAMETHYL-ALPHA-CHLORENAMINE
1-CHLORO-N,N,2-TRIMETHYLPROPENYLAMINE
(1-CHLORO-2-METHYLPROPENYL)DIMETHYLAMINE
1-CHLORO-N,N,2-TRIMETHYL-1-PROPENYLAMINE
1-chloro-N,N,2-trimethyl-1-Propen-1-amine
1-Chloro-N,N,2-trimethylpropenylamine ,98%
1-Chloro-N,N,2-triMethyl-1-propenylaMine 96%
1-Chloro-N,N,2-trimethylpropenylamine, 98.5+%
1-Chloro-N,N,2-triMethylpropenylaMine, 98.5+% 5ML | [Molecular Formula]
C6H12ClN | [MDL Number]
MFCD00800562 | [MOL File]
26189-59-3.mol | [Molecular Weight]
133.62 |
| Chemical Properties | Back Directory | [Appearance]
Colorless to yellow liquid | [Boiling point ]
129-130 °C(lit.)
| [density ]
1.01 g/mL at 25 °C(lit.)
| [refractive index ]
n20/D 1.453(lit.)
| [Fp ]
80 °F
| [storage temp. ]
Freezer (-20°C) | [solubility ]
Chloroform (Sparingly), Methanol (Slightly) | [form ]
Liquid | [pka]
4.27±0.70(Predicted) | [color ]
Colorless to yellow | [Stability:]
Hygroscopic, Moisture Sensitive, Unstable in Solution |
| Hazard Information | Back Directory | [Chemical Properties]
Colorless to yellow liquid | [Uses]
1-Chloro-N,N,2-trimethyl-1-propenylamine is an acid halogenation reagent developed by Ghosez, that enables the conversion of carboxylic acids into the corresponding chlorides under strictly neutral conditions. This method was successfully used in the total synthesis of caloporoside, roseophilin, (-)-enniatin B, (±)-epimeloscine and (±)-meloscine. | [Application]
1-Chloro-N,N,2-trimethylpropenylamine (TMCE) is used to mild halogenation of alcohols and acids under neutral conditions; conversion of N-protected amino acids into
peptides without racemization; coupling of acids and allylic alcohols with organometallics; [2 + 2]
cycloaddition synthons, more reactive than dimethylketene.
| [Preparation]
1-chloro-N,N,2-trimethylpropenylamine is conveniently prepared by the reaction of N,N,2-
trimethylpropanamide with Phosgene followed by dehydrochlorination of the intermediate α-chloroiminium
chloride (eq 1).[1,2] Recent unpublished results of our laboratory have shown that Phosphorus Oxychloride or di�or triphosgene can also be used for the preparation of TMCE.
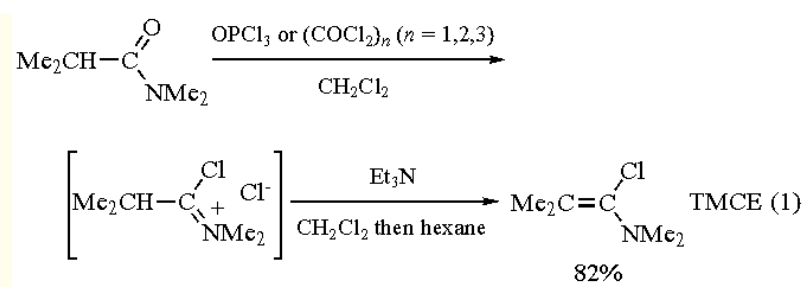 | [Synthesis Reference(s)]
Journal of the American Chemical Society, 94, p. 2869, 1972 DOI: 10.1021/ja00763a061
Organic Syntheses, Coll. Vol. 6, p. 282, 1988
Tetrahedron Letters, 25, p. 5043, 1984 DOI: 10.1016/S0040-4039(01)91114-1 | [storage]
1-chloro-N,N,2-propenylamines are thermally
stable. It must be transferred in the absence of moisture and stored under nitrogen in sealed tubes. In spite of
these precautions, light precipitates sometimes may form. 1-Iodo-N,N,2-trimethylpropenylamine is less stable and
should be used when freshly prepared. | [References]
1. Ghosez, L.; Marchand-Brynaert, J. In Advances in Organic Chemistry; Raphael, R. A.; Taylor, E. C.; Wynberg, H.,
Eds.; Interscience: New York, 1976; Part 1, pp 421-523.
2. (a) Haveaux, B.; Dekoker, A.; Rens, M.; Sidani, A. R.; Toye, J.; Ghosez, L. OS 1979, 59, 26. (b) Ghosez, L.; Koch, I.
Swiss Pat. 681 623A, 1993. |
|
|