| Identification | Back Directory | [Name]
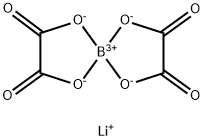
Lithium bis(oxalate)borate | [CAS]
244761-29-3 | [Synonyms]
LIBOB
CHEMLYTE BO M1
CHEMLYTE BO MX
lithium bis(oxalate)borate
LITHIUM BIS(OXALATO)BORATE
Lithium oxalate boronic acid
Lithium bis(ethanedioato)borate
LITHIUM BIS (OXALATE) BORATE(LiBOB)
Lithium bis(oxalate)borate ISO 9001:2015 REACH
Lithium bis(oxalate)borate LiBOB Lithium bis(ethanedioato)borate
Lithium bis-(oxalato)borate - LiBOB (sbg), standard battery grade
Borate(1-), bis(ethanedioato(2-)-kappao1,kappao2)-, lithium, (T-4)-
lithium,1,4,6,9-tetraoxa-5-boranuidaspiro[4.4]nonane-2,3,7,8-tetrone
LithiuM 2,3,7,8-tetraoxo-1,4,6,9-tetraoxa-5-boraspiro[4.4]nonan-5-uide
Borate(1-), bis(ethanedioato(2-)-kappao1,kappao2)-, lithium (1:1), (T-4)- | [EINECS(EC#)]
456-990-3 | [Molecular Formula]
C4BLiO8 | [MDL Number]
MFCD07776904 | [MOL File]
244761-29-3.mol | [Molecular Weight]
193.79 |
| Hazard Information | Back Directory | [Uses]
Lithium bis(oxalate)borate is a new and proprietary conductive salt for the use in high performance batteries like lithium batteries, lithium ion batteries and lithium polymer batteries. The new halide-free product may be used instead of traditional fluorinated compounds like LiPF6, LiBF4, Li-triflate, methanides, imides etc.
| [General Description]
We are committed to bringing you Greener Alternative Products, which adhere to one or more of The 12 Principles of Greener Chemistry. This product has been enhanced for energy efficiency. Find details here. | [Flammability and Explosibility]
Nonflammable | [Synthesis]
Yu et al. employed a solid-state reaction using oxalic acid, lithium hydroxide, and boric acid as starting materials for the first time. This was followed by a thermal treatment in the air and a recrystallization process to obtain LiBOB. In addition, LiBOB decomposes into Li2C2O3, B2O3, CO2, and CO at temperatures higher than 300 °C [1].
| [References]
[1] Ceren Zor. “Guide to Water Free Lithium Bis(oxalate) Borate (LiBOB).” The Journal of Physical Chemistry C 125 21 (2021): 11310–11317.
|
|
|