| Identification | Back Directory | [Name]
788Succinic acid | [CAS]
2389149-74-8 | [Synonyms]
788Succinic acid
TAK788 succinate
Mobocertinib succinate | [Molecular Formula]
C36H45N7O8 | [MDL Number]
MFCD32689632 | [MOL File]
2389149-74-8.mol | [Molecular Weight]
703.8 |
| Chemical Properties | Back Directory | [storage temp. ]
Store at -20°C | [solubility ]
DMSO : 25 mg/mL (35.52 mM; Need ultrasonic) | [form ]
Solid | [color ]
White to yellow | [InChIKey]
YXYAEUMTJQGKHS-UHFFFAOYSA-N | [SMILES]
C(C(=O)O)CC(=O)O.C(C1=CN=C(NC2C=C(NC(=O)C=C)C(N(C)CCN(C)C)=CC=2OC)N=C1C1=CN(C)C2=CC=CC=C12)(=O)OC(C)C |
| Hazard Information | Back Directory | [Description]
Mobocertinib (TAK-788) succinate is an orally active and irreversible EGFR/HER2 inhibitor. Mobocertinib succinate potently inhibits oncogenic variants containing activating EGFRex20ins mutations with selectivity over wild-type EGFR. Mobocertinib succinate can be used in NSCLC research. | [Uses]
Mobocertinib is an oral targeted therapy drug[1].Mobocertinib is used to treat a certain type of non-small cell lung cancer (NSCLC) that cannot be removed by surgery and has spread to other parts of the body either during or after treatment with platinum chemotherapy medications.
| [Mechanism of action]
Mobocertinib acts to inhibit EGFR exon 20 insertion mutations at a lower concentration than it does on wild-type proteins. | [Side effects]
Diarrhoea, rash, nausea, stomatitis, vomiting, decreased appetite, paronychia, fatigue, dry skin,musculoskeletal pain, increased amylase/lipase/creatinine, and decreased potassium/hemoglobin/magnesium/lymphocytes.
| [Synthesis]
Mobocertinib succinate is prepared by the reaction of Isopropyl 2-chloro-4-(1-methyl-1H-indol-3-yl)pyrimidine-5-carboxylate in the presence of an inert atmosphere.
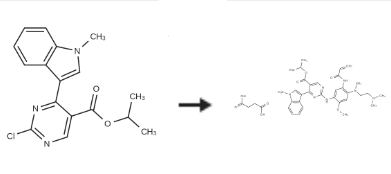 | [References]
[1] IMRANMOHD. Discovery, Development, Inventions, and Patent Trends on Mobocertinib Succinate: The First-in-Class Oral Treatment for NSCLC with EGFR Exon 20 Insertions.[J]. Biomedicines, 2021. DOI:10.3390/biomedicines9121938. |
|
|