| Identification | Back Directory | [Name]
METHYL 3-OXOTETRADECANOATE | [CAS]
22348-97-6 | [Synonyms]
Metyl lauroyl acetate
Methyl Lauroyl Acetate
Methyl b-ketomyristate
Methyl β-KetoMyristate
METHYL 3-OXOTETRADECANOATE
METHYL 3-KETOTETRADECANOATE
3-Oxomyristic acid methyl ester
3-Ketomyristic acid methyl ester
3-Oxotetradecanoic acid methyl ester
Tetradecanoic acid, 3-oxo-, methyl ester | [EINECS(EC#)]
244-929-1 | [Molecular Formula]
C15H28O3 | [MDL Number]
MFCD00673474 | [MOL File]
22348-97-6.mol | [Molecular Weight]
256.38 |
| Chemical Properties | Back Directory | [Appearance]
Yellow Crystalline Solid | [Melting point ]
29-30°C | [Boiling point ]
137-139 °C(Press: 0.9 Torr) | [density ]
0.926±0.06 g/cm3(Predicted) | [storage temp. ]
-20?C Freezer | [solubility ]
Chloroform (Slightly), Ethyl Acetate (Slightly), Methanol (Slightly) | [form ]
Solid | [pka]
10.65±0.46(Predicted) | [color ]
Light Yellow to Yellow | [LogP]
5.500 (est) |
| Hazard Information | Back Directory | [Chemical Properties]
Yellow Crystalline Solid | [Uses]
Methyl 3-Oxotetradecanoate (cas# 22348-97-6) is a compound useful in organic synthesis. |
| Questions And Answer | Back Directory | [Synthesis]
The carboxylic acid (13.8 g, 69 mmol, 1.0 equivalents) was dissolved in dry THF under an inert atmosphere and cooled to 0 oC. CDI (13.4 g, 83 mmol, 1.2 equivalents) was then added in small portions over several minutes. After 10 min, the reaction was allowed to warm slowly to room temperature and was then stirred for 1 h. In a separate flflask, monomethyl malonate (9.8g, 83 mmol, 1.2 equivalents) was dissolved in THF under an inert atmosphere and cooled to ?78 oC. To this solution was added dibutylmagnesium (1.0 M in heptane, 0.6 equivalents). A white solid formed instantly on addition of the base. After 10 min, the reaction was warmed to room temperature and stirred for 1 h. The acylimidazole was then added by cannula to the flflask containing the magnesium salt. The resulting slurry was stirred for 3 days. The reaction mixture was then concentrated on a rotary evaporator and the residue redissolved in EtOAc. The resulting solution was washed with 1.2 M HCl, saturated aqueous NaHCO3, and brine. The organic phase was dried over sodium sulfate, fifiltered, concentrated, and the residue purifified by flflash chromatography (7:1 hexanes:EtOAc) to give the β-keto ester (14.7g, 83%).
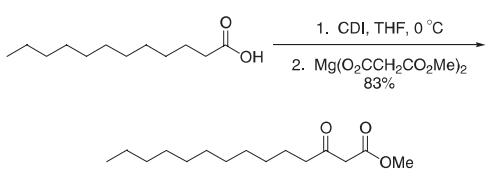
Reference: Durham, T. B.; Miller, M. J. J. Org. Chem. 2003, 68, 27–34. |
|
|