| Identification | Back Directory | [Name]
(S)-4-Methyl-2,5-oxazolidonedione | [CAS]
2224-52-4 | [Synonyms]
ALA-NCA
L-Ala-NCA
H-ALA-NCA
Alanine, NCA
L-Alanine NC
L-ALA-NCA(2224-52-4)
N-Carboxyalanine Anhydride
L-Alanine N-Carbxy anhydride
N-Carboxy-L-alanine anhydride
4-methyloxazolidine-2,5-quinone
(S)-4-METHYL-2,5-OXAZOLIDINEDIONE
(S)-4-Methyl-2,5-oxazolidonedione
(S)-4-METHYL-OXAZOLIDINE-2,5-DIONE
4-methyl-1,3-oxazolidine-2,5-dione
(4S)-4-Methyloxazolidine-2,5-dione
2,5-Oxazolidinedione,4-Methyl-, (4S)-
(4S)-4-Methyl-1,3-oxazolidine-2,5-dione | [EINECS(EC#)]
218-750-4 | [Molecular Formula]
C4H5NO3 | [MDL Number]
MFCD03411276 | [MOL File]
2224-52-4.mol | [Molecular Weight]
115.09 |
| Chemical Properties | Back Directory | [Melting point ]
92℃ | [alpha ]
+5.13°(25/D,c=6.4,dioxane) | [density ]
1.296 | [storage temp. ]
Inert atmosphere,Store in freezer, under -20°C | [solubility ]
DMSO (Very Slightly, Heated) | [form ]
Solid | [pka]
9.38±0.40(Predicted) | [color ]
White to Off-White | [InChI]
InChI=1S/C4H5NO3/c1-2-3(6)8-4(7)5-2/h2H,1H3,(H,5,7)/t2-/m0/s1 | [InChIKey]
DTETYCNJKAUROO-REOHCLBHSA-N | [SMILES]
O1C(=O)[C@H](C)NC1=O |
| Hazard Information | Back Directory | [Description]
(S)-4-Methyl-2,5-oxazolidonedione is also known as N-Carboxy anhydrides. The Fuchs-Farthing method, reported in 1922, was the only practical method for preparing (S)-4-Methyl-2,5-oxazolidonedione[1]. N-Carboxy anhydrides (NCAs) are important as α-amino acid building blocks for the synthesis of oligopeptides and nonpeptide compounds, and for the synthesis of polypeptides[2]. The polymerization of NCAs is the main process for the preparation of polypeptides, and its purity and side chains can significantly affect the synthesized polypeptides. NCAs polymerizations have been initiated using many different nucleophiles and bases, the most common being primary amines and alkoxide anions[3]. | [Uses]
(S)-4-Methyl-2,5-oxazolidonedione is a reagent used in the synthesis of poly(γ-benzyl-L-glutamate) and its block polypeptides with alanine, leucine and phenylalanine.
| [Uses]
N-Carboxyalanine Anhydride is a reagent used in the synthesis of poly(γ-benzyl-L-glutamate) and its block polypeptides with alanine, leucine and phenylalanine. | [Production Methods]
L-alanine (20.0 g, 0.224 mol) and triphosgene (53.4 g, 0.180 mol) were suspended in 400.0 mL of dry THF bubbled with nitrogen flux in a flame-dried three-neck flask. The mixture was stirred at 60 °C for 2 h before further bubbling with nitrogen flux for 30 min. After that, the solution was precipitated in 1000.0 mL of n-hexane and stored at -20 °C. The supernatant was removed, and the residues were collected and dissolved in 200.0 mL of ethyl acetate, before two washings with 100.0mL of ice-cold water and one washing with 100.0 mL of 0.5% NaHCO3 ice-cold aqueous solution. The organic phase was then dried over anhydrous MgSO4 and evaporated to obtain 15.5 g of L-Ala NCA ((S)-4-Methyl-2,5-oxazolidonedione). The yield of L-Ala NCA was 77.5%.
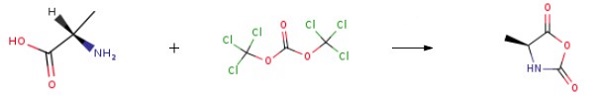
| [Synthesis Reference(s)]
The Journal of Organic Chemistry, 50, p. 2200, 1985 DOI: 10.1021/jo00212a042 | [References]
[1] Otake Y, et al. Rapid and Mild Synthesis of Amino Acid N-Carboxy Anhydrides
Using Basic-to-Acidic Flash Switching in a Micro-flow Reactor. Angewandte Chemie International Edition, 2018; 57: 11389-11393.
[2] Sch?fer G, et al. Synthesis of Sterically Hindered N-Acylated Amino Acids from N-Carboxyanhydrides. Organic Letters, 2014; 16: 1526–1529.[3] Imanishi Y, et al. Polymerization of α-amino acid N-carboxyanhydride in the presence of preformed poly(α-amino acid) - from chain effect to stereoselective polymerization. Pure and Applied Chemistry, 1981; 53. |
|
|