| Identification | Back Directory | [Name]
Cyproterone | [CAS]
2098-66-0 | [Synonyms]
SH-881
SH-80881
cyproteron
CYPROTERONE
Cyproterone-d5
ro-17-hydroxy-
Cyproterone USP/EP/BP
Cyproterone Acetate Impurity F
Cyproterone Acetate EP Impurity F
Cyproterone Acetate 427-51-0 /Base
Cyproterone Acetate IMpurity F (Cyproterone)
Cyproterone Acetate EP Impurity F (Cyproterone)
1,2α-Methylene-6-chloro-6-17α-hydroxyprogesterone
6-Chloro-6-dehydro-17α-hydroxy-1,2α-Methyleneprogesterone
6-Chloro-17-hydroxy-1α,2α-Methylenepregna-4,6-diene-3,20-dione
Cyproterone Acetate Impurity 6(Cyproterone Acetate EP Impurity F)
6-chlor-delta(sup6)-1,2-alpha-methylen-17-alpha-hydroxyprogesteron
4,6-PREGNADIEN-6-CHLORO-1-ALPHA, 2-ALPHA-METHYLENE-17-OL-3,20-DIONE
(1β,2β)-6-Chloro-1,2-dihydro-17-hydroxy-3'H-cyclopropa[1,2]pregna-1,4
3’h-cyclopropa(1,2)pregna-1,4,6-triene-3,20-dione,6-chloro-1-beta,2-beta-dihyd
6-Chloro-1β,2β-dihydro-17-hydroxy-3'H-cyclopropa[1,2]pregna-4,6-diene-3,20-dione
6-chloro-1b,2b-dihydro-17-hydroxy-3'h-cyclopropa(1,2)pregna-1,4,6-triene-3,20-dione
3'H-Cyclopropa[1,2]pregna-1,4,6-triene-3,20-dione, 6-chloro-1,2-dihydro-17-hydroxy-, (1β,2β)- | [Molecular Formula]
C22H27ClO3 | [MDL Number]
MFCD00869226 | [MOL File]
2098-66-0.mol | [Molecular Weight]
374.9 |
| Chemical Properties | Back Directory | [Melting point ]
208-210°C | [Boiling point ]
521.0±50.0 °C(Predicted) | [density ]
1.29±0.1 g/cm3(Predicted) | [storage temp. ]
Refrigerator | [solubility ]
Chloroform (Slightly), Methanol (Slightly) | [form ]
Solid | [pka]
12.86±0.70(Predicted) | [color ]
Off-White to Yellow |
| Hazard Information | Back Directory | [Chemical Properties]
White Solid | [Uses]
An antiandrogen, suppresses Testosterone and its metabolites. Derivatives of Cyproterone are administered to patients suffering from hypersexuality and to help facilitate the sexual transformation of male-to-female transsexuals. | [Uses]
insecticide | [Definition]
ChEBI: Cyproterone is a 20-oxo steroid, a 17alpha-hydroxy steroid, a chlorinated steroid, a 3-oxo-Delta(4) steroid and a tertiary alpha-hydroxy ketone. It has a role as an androgen antagonist. It derives from a hydride of a pregnane. | [World Health Organization (WHO)]
Cyproterone was introduced in the late sixties. It is an orallyactive
anti-androgen with competitive inhibitory effects on androgen-sensitive
target organs. It also has anti-gonadotropic and progestative properties. In 1995
the drug was found to have a hepatotoxic effect. | [Synthesis]
Cyproterone, 6-chloro-17|á-hydroxy-1|á,2|á-methylenpregna-4,6-dien-3,20-
dione acetate (29.2.14), is made from 17|á-hydroxyprogesterone acetate (28.3.36).
Dehydrating this using chloranyl (tetrachloro-p-benzoquinone) results in formation of an
additional double bond at position C6¨CC7 (29.2.8), and subsequent dehydration using sele�nium dioxide to form the corresponding divinyl ketone, 17|á-acetoxy-1,4,6-pregnatrien-
3,20-dione (29.2.9). Reacting this with diazomethane results in a 1,3-dipolar addition
reaction at C1¨CC2 of the double bond of the steroid system, which forms a derivative of
dihydropyrazole (29.2.10). This compound cleaves when reacted with chloric acid, releas�ing nitrogen molecules and forming a cyclopropane derivative (29.1.11). Next, the double
bond at C6¨CC7 is selectively oxidized by benzoyl peroxide, and the resulting epoxide
(29.2.12) undergoes a reaction with hydrochloric acid in acetic acid, resulting in the forma�tion of chlorine and its subsequent dehydration, and a simultaneous opening of the cyclo�propane ring, forming the compound (29.2.13). Heating this in collidine results in
intramolecular alkylation, causing cyclization into a cyclopropane ring, which forms cypro�terone (29.2.14).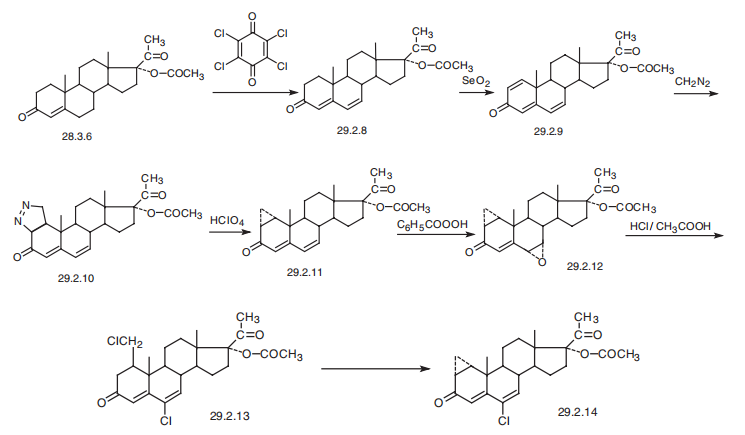
|
|
| Company Name: |
LGM Pharma
|
| Tel: |
1-(800)-881-8210 |
| Website: |
www.lgmpharma.com |
|