| Identification | Back Directory | [Name]
3-(6-Chloro-5-fluoropyrimidin-4-yl)-2-(2,4-difluorophenyl)-1-(1H-1,2,4-triazol-1-yl)butan-2-ol hydrochloride | [CAS]
188416-20-8 | [Synonyms]
Voriconazole
PI-5
-2-(2,4-difluorophenyl)
butan-2-ol hydrochloride
4-difluorophenyl)-1-(1H-1
-1-(1H-1,2,4-triazol-1-yl)
Voriconazole Impurity 76 HCl
3-(6-Chloro-5-fluoropyrimidin-4-yl)
4-triazol-1-yl)butan-2-ol hydrochloride
3-(6-Chloro-5-fluoropyrimidin-4-yl)-2-(2
3-(6-Chloro-5-fluoropyrimidin-4-yl)-2-(2,4-difluorophen
(2r,3s/2s,3r)-3-(4-chloro-5-fluoro-6-pyrimidinyl)-2-(2,4-dif...
(2R,3S/2S,3R)-3-(4-Chloro-5-fluoro-6-pyriMidinyl)-2-(2,4-difluorophenyl)-1-(1H-1,2,4-
3-(6-Chloro-5-fluoropyrimidin-4-yl)-2-(2,4-difluorophenyl)-1-(1H-1,2,4-triazol-1-yl)butan-2-ol hy
3-(6-chloro-5-fluoropyrimidin-4-yl)-2-(2,4-difluorophenyl)-1-(1,2,4-triazol-1-yl)butan-2-ol,hydrochloride
3-(6-Chloro-5-fluoropyrimidin-4-yl)-2-(2,4-difluorophenyl)-1-(1H-1,2,4-triazol-1-yl)butan-2-ol hydrochloride
3-(6-CHLORO-5-FLUOROPYRIMIDIN-4-YL)-2,(2,4-DIFLUORO PHENYL)-1(1H-1,2,4-TRIAZOL-1-YL)BUTANE-2-OL HYDRO CHLORIDE
3-(6-CHLORO-5-FLUOROPYRIMIDIN-4-YL)-2,(2,4-DIFLUORO PHENYL) - 1-(1H-1,2,4-TRIAZOLE-1-YL)BUTAN-2-OL HYDROCHLORIDE
(2R,3S/2S,3R)-3-(4-chloro-5-fluoro-6-pyrimidinyl)-2-(2,4-
difluorophenyl)-1-(1H-1,2,4-triazol-1-yl)-2-butanol HCL
3-(4-chloro-5-fluoropyrimidin-6-yl)-2-(2,4-difluorophenyl)-1-(1H-1,2,4-triazol-1-yl)butan-2-ol hydrochloride salt
( 2R,3S/2S,3R )-3-(4-chloro-5-fluoro-6-pyriMidinyl)-2-(2,40difluorophenyl)-1-(1H-1,2,4-triazol-1-yl)-2-butanol HCl
3-(6-Chloro-5-fluoropyrimidin-4-yl)-2-(2,4-difluorophenyl)-1-(1H-1,2,4-triazol-1-yl)butan-2-ol hydrochlorideChemicalBook
(2R,3S/2S,3R)-3-(6-Chloro-5-fluoropyrimidin-4-yl)-2-(2,4-difluorophenyl)-1-(1H-1,2,4-triazol-1-yl)butan-2-ol hydrochloride
(2R,3S/2S,3R)-3-(5-fluoro -6-chloro-4-pyrimidinyl)-2-(2,4-difluorophenyl)-1-(1H-1,2,4-triazol-1-yl)-2-butanol Hydrochloride
(αR,βS)-rel-6-chloro-α-(2,4-difluorophenyl)-5-fluoro-β-Methyl-α-(1H-1,2,4-triazol-1-ylMethyl)-4-pyriMidineethanol Monohydrochloride
(2R,3S/2S,3R)-3-(4-Chloro-5-fluoro-6-pyriMidinyl)-2-(2,4-difluorophenyl)-1-(1H-1,2,4-triazol-1-yl)-2-butanol hydrochloride (for Voriconazole) | [Molecular Formula]
C16H13ClF3N5O.ClH | [MDL Number]
MFCD11113103 | [MOL File]
188416-20-8.mol | [Molecular Weight]
420.221 |
| Chemical Properties | Back Directory | [storage temp. ]
Sealed in dry,Store in freezer, under -20°C | [solubility ]
DMSO (Slightly), Methanol (Slightly) | [form ]
Solid | [color ]
White to Off-White | [Stability:]
Hygroscopic |
| Hazard Information | Back Directory | [Uses]
3-(6-Chloro-5-fluoropyrimidin-4-yl)-2-(2,4-difluorophenyl)-1 can be used as organic synthesis intermediates and pharmaceutical intermediates, mainly used in laboratory research and development processes and chemical production processes. | [Synthesis]
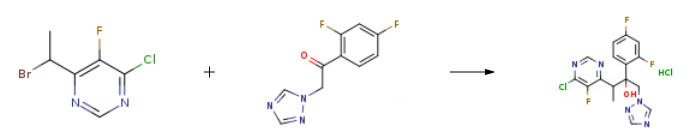
Under nitrogen atmosphere, 9.35 g of zinc powder and 0.47 g of lead are introducedto 53 mL of dry tetrahydrofuran (THF) and the mixture is agitated for 3 hours under reflux. The reaction mixture is cooled to 25??C and agitated continuously for 16 hours. In a separate container, 7.42 g of iodine is dissolved into 21 mL of dry tetrahydrofuran (THF) and the resultant solution is added dropwise to the reaction mixture over 80 minutes. Next, the reaction mixture is warmed to 45??C and then cooled to 0??C.[119] At room temperature, 6.53 g of 1-(2,4-difluorophenyl)-2-(1H-1,2,4-triazol-1-yl)ethanone and 7.01 g of 6-(1-bromo ethyl)-4-chloro-5-fluoropyrimidine are dissolved into 53 mL of tetrahydrofuran (THF) and the resultant solution is added dropwise gradually to the reaction mixture, while maintaining the reaction temperature at 5??C or lower. The reaction mixture is warmed to 25??C and 8.84 g of glacial acetic acid dissolved in 84 mL of purified water is added dropwise to the reaction mixture. The solid metal residueis removed by filtering, the solvent is distilled off under reduced pressure, and the reaction product is extracted twice with 84 mL of ethyl acetate (EA). The extract is washed with 3.22 g of disodium ethylenediamine tetraacetate dihydrate dissolved in 161 mL of purified water, and further washed with 30 mL of saline. The organic layer is concentrated to a final volume of 56 mL, and a solution containing 1.2 g of hydrochloric acid in 6 mL of isopropanol is added dropwise thereto at 25??C. The resultant crystals are filtered, washed with 5 mL of EA, and dried under reduced pressure for 12 hours at 50??C to obtain 5.99 g of the target compound (yield 48.7%, purity 96.2%, HPLC, detected at a wavelength of 256 nm, 18C 4.6 X 250 mm, mobile phase 60% ACN, flow rate 1 mL/min).[120] 1H-NMR (200MHz, DMSO-d6) |? (ppm) : 8.85(1H), 8.731H), 7.93(1H), 7.28~7.16(2H), 6.95~6.89(1H), 5.83(1H), 4.81(1H), 4.54(1H), 3.92(1H), 1.13(3H). |
|
|