| Identification | Back Directory | [Name]
PALLADIUM (I) TRI-TERT-BUTYLPHOSPHINE BROMIDE | [CAS]
185812-86-6 | [Synonyms]
Pd-113
Di-μ
broMopalladiuM
[Pd(μ-Br)t-Bu3P]2
PALLADIUM (I) TRI-TERT-BUTYLPHOSPHINE BROMIDE
Dibromobis(tri-tert-butylphosphine)dipalladium
Bromo(tri-tert-butylphosphine)palladium(I)dimer
DI-BROMOBIS(TRI-T-BUTYLPHOSPHINO)DIPALLADIUM (I)
Dibromobis(tri-tert-butylphosphine)dipalladium(I)
Di-mu-bromobis(tri-t-butylphosphino)dipalladium(I)
DI--BROMOBIS(TRI-TERT-BUTYLPHOSPHINO)DIPALLADIUM(I)
PALLADIUM (I) TRI-TERT-BUTYLPHOSPHINE BROMIDE, DIMER
Di-^M-broMobis(tri-tert-butylphosphine)dipalladiuM(I)
PalladiuM, di-M-broMobis[tris(1,1-diMethylethyl)phosphine]di-,(Pd-Pd)
Dibromobis(tri-tert-butylphosphino)dipalladium(I), Palladium(I) tri-tert-butylphosphine bromide, dimer
-bromobis(tri-tert-butylphosphine)dipalladium/ Pd (I) dimer Reasonably stable for packaging and transfer. | [Molecular Formula]
C24H54Br2P2Pd2 | [MDL Number]
MFCD04114019 | [MOL File]
185812-86-6.mol | [Molecular Weight]
777.28 |
| Chemical Properties | Back Directory | [Appearance]
Greenish blue | [storage temp. ]
?20°C | [form ]
crystal | [color ]
dark-green | [Water Solubility ]
Soluble in benzene and tolueneSoluble in tetrahydrofuran, toluene, dichloromethane and chloroform. Insoluble in alcohols and water. | [Sensitive ]
Air Sensitive |
| Hazard Information | Back Directory | [Chemical Properties]
Greenish blue | [Uses]
Coupling reactions. Will activate aryl chloride and sterically hindered or electron rich aryl/vinyl bromides and iodides. Especially active in difficult aminations.Di-μ-bromobis(tri-tert-butylphosphine)dipalladium(I) is used as a catalyst for Suzuki coupling, Negishi coupling and Buchwald-hartwig amination reactions. It is used as a catalyst for C-C, C-N and C-S bond formation, gamma-arylation of alfa,beta-unsaturated esters and diastereoselective arylation of 4-substituted cyclohexyl esters. It is also involved in aromatic halide substitution reactions. |
| Questions And Answer | Back Directory | [Reactions]
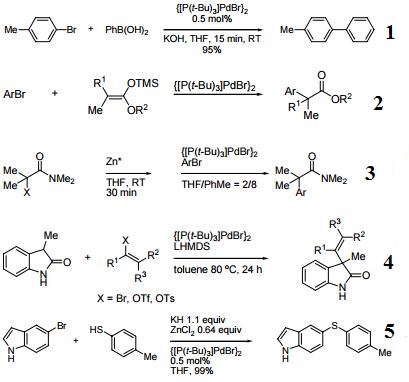
- Palladium catalyst for rapid room temperature alkylation of unactivated hindered aryl bromides with arylboronic acids.
- Aryl bromide - silyl ketene acetal coupling.
- Catalyst for intermolecular α-arylation of zinc amide enolates.
- Catalyst for α-vinylation of carbonyl compounds.
- Catalyst for thiol coupling of heteroaromatic aryl bromides.
|
|
|