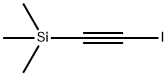| Identification | Back Directory | [Name]
1-IODO-2-(TRIMETHYLSILYL)ACETYLENE 97 | [CAS]
18163-47-8 | [Synonyms]
(iodoethynyl)triMethylsilane
2-Iodoethynyl(Trimethyl)Silane
Silane, (2-iodoethynyl)trimethyl-
1-Iodo-2-(trimethylsilyl)acetylene
1-IODO-2-(TRIMETHYLSILYL)ACETYLENE 97
1-IODO-2-(TRIMETHYLSILYL)ACETYLENE 97 ISO 9001:2015 REACH | [Molecular Formula]
C5H9ISi | [MDL Number]
MFCD00274201 | [MOL File]
18163-47-8.mol | [Molecular Weight]
224.112 |
| Chemical Properties | Back Directory | [Boiling point ]
130 °C(lit.)
| [density ]
1.46 g/mL at 25 °C(lit.)
| [refractive index ]
n20/D 1.511(lit.)
| [Fp ]
119 °F
| [storage temp. ]
2-8°C | [solubility ]
sol organics (THF, Et2O, pyridine, DCM, CHCl3,
DMF). | [form ]
clear liquid | [color ]
Colorless to Red to Green | [Specific Gravity]
1.46 | [Hydrolytic Sensitivity]
4: no reaction with water under neutral conditions | [Sensitive ]
Light Sensitive | [CAS DataBase Reference]
18163-47-8 |
| Hazard Information | Back Directory | [Physical properties]
bp 96 °C, 55 °C/20 mmHg; n20
D 1.5110;
d 1.46 g cm?3, fp 48°C. | [Uses]
(Iodoethynyl)trimethylsilane is widely used as alkyne coupling reagent predominantly used in conjunction with
organocopper species or palladium catalysis.(Iodoethynyl)trimethylsilane has been largely
confined to aryl- and vinylcopper coupling, where it serves well
since the reverse procedure, i.e. coupling with alkynylcopper
reagents, is not straightforward due to the lack of reactivity of
alkynyl moieties bound to copper. | [Uses]
1-Iodo-2-(trimethylsilyl)acetylene may be used to investigate the reactivity of diamagnetic cobalt(I) complex, Tol-BDI((2-pp)2)Co [Tol-BDI((2-pp)2)H = 2-(4-tolyl)-1,3-bis(2-isopropylpyridyl)propenediimine]. It may be used for the synthesis of 1-(4-trimethylsilanylbuta-1,3-diynyl)pyrene, via Pd-coupling with 1-iodo-2-trimethylsilylacetylene. | [Preparation]
All involve iodination of trimethylsilylacetylenes.
Direct iodination of metalated (lithium or magnesium)
trimethylsilylacetylene has been largely supplanted
due to the modest yields obtained. Utilizing trimethylsilylacetylene
and bis(trimethylsilyl) peroxide in the presence
of copper(I) iodide gives good yields. Substituting zinc
iodide requires using the lithium acetylide. Copper(I) iodide
(0.05 equiv.) in the presence of iodine, sodium carbonate, and
a phase-transfer catalyst gives good yields without having to
use n-butyllithium or a Grignard reagent to first deprotonate
the trimethylsilylacetylene. The most popular procedure uses
bis(trimethylsilyl)acetylene, treating it with iodine monochloride
in dichloromethane at 25°Cto obtain excellent yields of
(iodoethynyl)trimethylsilane. |
|
|