| Identification | Back Directory | [Name]
Butanedioic acid 4-amino-5-chloro-2,3-dihydro-N-[1-(3-methoxypropyl)-4-piperidinyl]-7-benzofurancarboxamide | [CAS]
179474-85-2 | [Synonyms]
R-108512
RESOLOR; R-108512
Resolor Succinate
PRUCALOPRIDE SUCCITE
Priucapride succinate
Prucalopride Impurity
Prucalopride Succinat
Prucalopride Succinate
Prucalopride Succinate WS
Prucalopride Succinate API
PRUCALOPRIDE INTERMEDIATES
7-cyclopenta[b]pyrancarboxamide
Prucalopride Succinate USP/EP/BP
TIANFU-CHEM - Prucalopride Succinate
Prucalopride Succinate (R-108512, R-93877)
Butanedioic acid 4-amino-5-chloro-2,3-dihydro-N-[1-(3-methox...
Prucalopride succinate, 98.5%, a selective high affinity 5-HT4 receptor agonist
4-amino-5-chloro-N-(1-(3-methoxypropyl)piperidin-4-yl)-2,3-dihydrobenzofuran-7-carboxamide succinate
4-Amino-5-chloro-2,3-dihydro-N-[1-(3-methoxypropyl)-4-piperidinyl]-7-benzofurancarboxamide Butanedioic Acid
Butanedioic acid 4-amino-5-chloro-2,3-dihydro-N-[1-(3-methoxypropyl)-4-piperidinyl]-7-benzofurancarboxamide
4-amino-5-chloro-N-[1-(3-methoxypropyl)piperidin-4-yl]-2,3-dihydro-1-benzofuran-7-carboxamide,butanedioicaci
4-amino-5-chloro-N-[1-(3-methoxypropyl)piperidin-4-yl]-2,3-dihydro-1-benzofuran-7-carboxamide,butanedioic acid
Prucalopride Succinate
4-amino-5-chloro-N-(1-(3-methoxypropyl)piperidin-4-yl)-2,3
-dihydrobenzofuran-7-carboxamide succinate
Butanedioic acid, compd. with 4-amino-5-chloro-2,3-dihydro-N-[1-(3-methoxypropyl)-4-piperidinyl]-7-benzofurancarboxamide (1:1)
Prucalopride Succinate 13CD3Q: What is
Prucalopride Succinate 13CD3 Q: What is the CAS Number of
Prucalopride Succinate 13CD3 | [EINECS(EC#)]
680-639-5 | [Molecular Formula]
C18H26ClN3O3.C4H6O4 | [MDL Number]
MFCD00948731 | [MOL File]
179474-85-2.mol | [Molecular Weight]
485.958 |
| Chemical Properties | Back Directory | [Melting point ]
>196°C (dec.) | [storage temp. ]
Sealed in dry,Room Temperature | [solubility ]
DMSO (Slightly), Methanol (Slightly) | [form ]
Solid | [color ]
White to Pale Brown | [InChIKey]
QZRSNVSQLGRAID-UHFFFAOYSA-N | [SMILES]
C(O)(=O)CCC(O)=O.O1C2=C(C(NC3CCN(CCCOC)CC3)=O)C=C(Cl)C(N)=C2CC1 |
| Hazard Information | Back Directory | [Chemical Properties]
Butanedioic acid 4-amino-5-chloro-2,3-dihydro-N-[1-(3-methoxypropyl)-4-piperidinyl]-7-benzofurancarboxamide is Off-White to Pale Brown Solid
| [Uses]
Butanedioic acid 4-amino-5-chloro-2,3-dihydro-N-[1-(3-methoxypropyl)-4-piperidinyl]-7-benzofurancarboxamide is a selective 5-HT4 receptor agonist used effective for chronic constipation, but is not currently approved in the U.S. Prucalopride is approved for the treatment of chronic constipation in Europe.
| [Brand name]
Motegrity, Resolor, Resotran | [Mechanism of action]
The succinate form of prucalopride is an orally bioavailable dihydrobenzofurancarboxamide and selective serotonin (5-HT4) receptor agonist with pro-gastrointestinal motility activity. Upon oral administration, procalcitonin specifically targets, binds and stimulates 5-HT4 receptors. This alters the pattern of colonic motility and stimulates large amounts of peristalsis in the colon. This normalises bowel movements and relieves chronic constipation. In addition, by increasing peristalsis in the oesophagus and stomach, prucalopride also relieves symptoms associated with inhalation. | [Clinical Use]
Prucalopride succinate(179474-85-2
), a first-in-class dihydrobenzofurancarboxamide, is a selective serotonin (5-
HT4) receptor agonist. The drug, marketed under the brand name Resolor?, possesses
enterokinetic activity and was developed by the Belgian-based pharmaceutical firm Movetis.
Prucalopride alters colonic motility patterns via serotonin 5-HT4 receptor stimulation, triggering the
central propulsive force for defecation.
| [Side effects]
The most common adverse reactions to Prucalopride Succinate(179474-85-2
) include: headache, nausea, diarrhoea and abdominal pain, loss of appetite, dizziness, vomiting, digestive disturbances (dyspepsia), windiness, abnormal bowel sounds, and tiredness. Other side effects that may come out below are: tremor, rapid heartbeat, rectal bleeding, increased urination (pollenuria), fever, overall general feeling of malaise, flatulence. Severe allergic reactions are rare and manifest as hives or lumps, itching, rash���,redness of the skin, facial swelling, and chest tightness. Contact your doctor if any of these serious adverse reactions occur.
| [Synthesis]
The preparation of prucalopride succinate begins with
the commercially available salicylic aniline 124. Acidic esterification, acetylation of the
aniline nitrogen atom, and ambient-temperature chlorination via sulfuryl chloride (SO2Cl2) converted
aminophenol 124 to acetamidoester 125 in 83% yield over the course of three steps. An
unique set of conditions involving sodium tosylchloramide (chloramine T) trihydrate and sodium iodide were then employed to convert 125 to o-phenolic iodide 126, which then underwent sequential
Sonogashira/cyclization reaction utilizing TMS-acetylene with tetramethylguanidine (TMG) in the
presence of silica gel to furnish the benzofuran progenitor of 127. Hydrogenation of this
intermediate benzofuranyl Sonagashira product saturated the 2,3-benzofuranyl bond while leaving the
chlorine atom intact, ultimately delivering dihydrobenzofuran 127 in excellent yield for the two step
sequence. Base-induced saponification and acetamide removal gave rise to acid 128. This acid was
activated as the corresponding mixed anhydride and treated with commercial piperidine 129 to construct
prucalopride which was stirred at room temperature for 24 hours in ethanolic succinic acid to provide
prucalopride succinate (XI). The yield for the formation of the salt was not provided.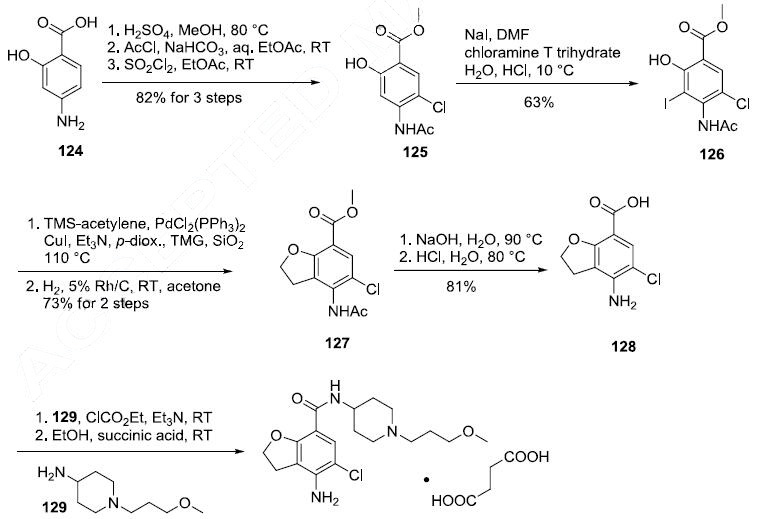
|
|
|