| Identification | Back Directory | [Name]
NAGLY | [CAS]
179113-91-8 | [Synonyms]
NAGLY
NAGly, CID 5283389
Arachidonyl Glycine
ARACHIDONOYL GLYCINE
N-ARACHIDONYL GLYCINE
N-ARACHIDONOYL GLYCINE
N-Arachidonoyl glycine (solution in ethanol)
N-(1-OXO-5Z,8Z,11Z,14Z-EICOSATETRAENYL)GLYCINE
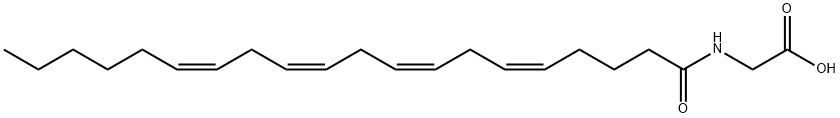
Glycine, N-[(5Z,8Z,11Z,14Z)-1-oxo-5,8,11,14-eicosatetraen-1-yl]- | [Molecular Formula]
C22H35NO3 | [MDL Number]
MFCD03791313 | [MOL File]
179113-91-8.mol | [Molecular Weight]
361.52 |
| Chemical Properties | Back Directory | [Boiling point ]
560.9±50.0 °C(Predicted) | [density ]
0.985±0.06 g/cm3(Predicted) | [storage temp. ]
−20°C | [solubility ]
DMSO: >5 mg/mL, soluble | [form ]
waxy solid | [pka]
3.58±0.10(Predicted) | [color ]
off-white | [Sensitive ]
Air Sensitive | [Stability:]
Stable for 2 years from date of purchase as supplied. Product is subject to oxidation. Solutions in degassed DMSO may be stored at -20°C for up to 1 month. |
| Hazard Information | Back Directory | [Description]
Arachidonoyl glycine (N-arachidonyl glycine; NAGly) has been isolated from cell cultures treated with arachidonoyl ethanolamide (AEA; anandamide), from extracts of mammalian brain, and has also been synthesized as an analog of AEA for structure/activity testing. NAGly may be produced endogenously via oxidation of AEA, or by transacylation of arachidonoyl CoA. NAGly is reported to have analgesic activities in whole animal experiments. Since it seems to be a very poor ligand for the CB1 receptor, these effects are probably mediated via other signaling pathways. | [Uses]
An endogenous anandamide-like compound; also acts as a T-type Ca2+ channel blocker and an endogenous GLYT2 inhibitor. | [Uses]
Arachidonoyl glycine (N-arachidonyl glycine; NAGly) has been isolated from cell cultures treated with arachidonoyl ethanolamide (AEA; anandamide), from extracts of mammalian brain, and has also been synthesized as an analog of AEA for structure/activity testing. NAGly may be produced endogenously via oxidation of AEA, or by transacylation of arachidonoyl CoA. NAGly is reported to have analgesic activities in whole animal experiments. Since it seems to be a very poor ligand for the CB1 receptor, these effects are probably mediated via other signaling pathways.[Cayman Chemical] | [Definition]
ChEBI:N-arachidonoylglycine is biologically active derivative of anandamide It is a N-acylglycine and a fatty amide. It is functionally related to an arachidonic acid. It is a conjugate acid of a N-arachidonoylglycinate. | [Biological Activity]
Endogenous anandamide-like compound. Lacks affinity for CB 1 receptors (K i > 10 μ M), VR1 receptors (EC 50 > 10 μ M) and anandamide transporters (IC 50 > 50 μ M) but causes hot-plate analgesia in mice when given orally, and suppresses tonic inflammatory pain. Also endogenous GlyT2 inhibitor. | [storage]
Store at -80°C | [References]
1) Huang et al. (2001), Identification of a new class of molecules, the arachidonyl amino acids, and characterization of one member that inhibits pain; J. Biol. Chem., 276 42639
2) McHugh et al. (2012), Δ(9)-Tetrahydrocannabinol and N-arachidonylglycine are full agonists at GPR18 receptors and induce migration in human endometrial HEC-1B cells; Br. J. Pharmacol., 165 2414
3) McHugh et al. (2012), siRNA knockdown of GPR18 receptors in BV-2 microglia attenuates N-arachidonyl glycine-induced cell migration; J. Mol. Signal., 7 10
4) Takenouchi et al. (2012), N-arachidonyl glycine induces macrophage apoptosis via GPR18; Biochem. Biophys. Res. Commun., 418 366
5) Burstein et al. (2011), Resolution of inflammation by N-arachidonoylglycine; J. Cell Biochem., 112 3227
6) Console-Bram et al. (2017), N-arachidonoyl glycine, another endogenous agonist of GPR55; Biochem. Biophys. Res. Commun., 490 1389 [FOCUS CITATION] |
|
| Company Name: |
Alfa Chemistry
|
| Tel: |
1-516-6625404 |
| Website: |
https://www.alfa-chemistry.com |
| Company Name: |
BOC Sciences
|
| Tel: |
|
| Website: |
https://www.bocsci.com |
| Company Name: |
Energy Chemical
|
| Tel: |
021-58432009 400-005-6266 |
| Website: |
http://www.energy-chemical.com |
|