| Identification | Back Directory | [Name]
MONOLINURON | [CAS]
1746-81-2 | [Synonyms]
ARESIN
Afesin
Arezin
Arezine
ARRESIN
hoe2747
Premalin
HOE 2747
Monorotox
Aresin(TM)
MONOLINURON
Monolinuron 0
Monolinuron-D6
3-(4-Chlorophenyl)
Monolinuron Standard
-1-methoxy-1-methylurea
monolinuron (bsi,iso,wssa)
Monolinuron @100 μg/mL in MeOH
Monolinuron 250mg [1746-81-2]
Linuron Impurity 3 (Monolinuron)
MONOLINURON PESTANAL (3-(4-CHLORO- PHENY
1-Methoxy-1-methyl-3-(4-chlorophenyl)urea
3-(4-CHLOROPHENYL)-1-METHOXY-1-METHYLUREA
3-(p-chlorophenyl)-1-methoxy-1-methyl-ure
n-(4-chlorophenyl)-n’-methoxy-n-methylurea
N'-(4-Chlorophenyl)-N-methoxy-N-methylurea
N-(4-Chlorophenyl)-N'-methoxy-N-methylurea
n’-(4-chlorophenyl)-n-methoxy-n-methyl-ure
N’-(4-chlorophenyl)-N-methoxy-N-methylurea
3-(p-Chlorophenyl)-1-methoxy-1-methyl urea
urea,3-(p-chlorophenyl)-1-methoxy-1-methyl-
Urea, 3-(p-chlorophenyl)-1-methoxy-1-methyl-
Urea,N'-(4-chlorophenyl)-N-Methoxy-N-Methyl-
3-(4-Chlorphenyl)-1-methoxy-1-methylharnstoff
UREA,3-(PARA-CHLOROPHENYL)-1-METHOXY-1-METHYL-
monolinuron (ISO) 3-(4-chlorophenyl)-1-methoxy-1-methylurea | [EINECS(EC#)]
217-129-5 | [Molecular Formula]
C9H11ClN2O2 | [MDL Number]
MFCD00055264 | [MOL File]
1746-81-2.mol | [Molecular Weight]
214.65 |
| Chemical Properties | Back Directory | [Melting point ]
81.5℃ | [density ]
1.3575 (rough estimate) | [refractive index ]
1.5790 (estimate) | [Fp ]
100 °C | [storage temp. ]
0-6°C | [form ]
neat | [pka]
12.80±0.70(Predicted) | [color ]
White to Light yellow to Light orange | [Water Solubility ]
0.58g/L(room temperature) | [BRN ]
2212523 | [InChI]
InChI=1S/C9H11ClN2O2/c1-12(14-2)9(13)11-8-5-3-7(10)4-6-8/h3-6H,1-2H3,(H,11,13) | [InChIKey]
LKJPSUCKSLORMF-UHFFFAOYSA-N | [SMILES]
N(OC)(C)C(NC1=CC=C(Cl)C=C1)=O | [EPA Substance Registry System]
Monolinuron (1746-81-2) |
| Hazard Information | Back Directory | [Uses]
The certified reference material (CRM) is intended to be used as a calibrant for chromatography and other analytical techniques.
Monolinuron CRM may also be used as given below:
- Determination of 252 pesticides extracted from surface water samples by solid-phase extraction (SPE) using liquid chromatography quadrupole-orbitrap high-resolution tandem mass spectrometry
- Extraction of 13 pesticides from four groundwater and three river water samples using silica-based MSU-1 mesoporous solid as a sorbent for their solid-phase extraction (SPE) followed by ultra-performance liquid chromatography-triple quadrupole-mass spectrometric (UHPLC- QqQ-MS/MS) determination
- Development and validation of a UHPLC-diode array detection (DAD) method for simultaneous determination of urea and tebuthiuron herbicides in four fresh vegetable samples
- Multi-residue analysis of 50 herbicides in 51 grain samples of soybean, corn, and whe at by ultra-performance liquid chromatography-electrospray ionization-mass spectrometry (UPLC-ESI-MS)
- Extraction of 109 pesticide residues from different chemical classes from 345 tomato samples by modified QuEChERS method and their determination using liquid chromatography-tandem mass spectrometry (LC-MS/MS)
- Simultaneous analysis of 238 pesticides and 78 veterinary drugs in 80 bovine milk samples using ultra-fast liquid chromatography-tandem mass spectrometry (UFLC-MS/MS) and gas chromatography-tandem mass spectrometry (GC-MS/MS)
| [Definition]
ChEBI: Monolinuron is a member of ureas. | [Reactions]
Nitrite or nitrate induced-photolysis of monolinuron (a phenyl urea herbicide) using the 300–450 nm light excitation gives rise to various photo-products. The direct photolysis of monolinuron proceeds via two main pathways yielding, respectively, 3-(4-chlorophenyl)-1-methylurea, which results from demethoxylation of the N-terminus substituted functional group and 3-(4-hydroxyphenyl)-1-methoxy-1- methylurea obtained by photohydrolysis of the C-Cl bond[1].
Degradation pathways of monolinuron by direct and nitrite (or nitrate) induced photolysis. Specific photoproducts: circled numbers (direct); underlined numbers (induced):
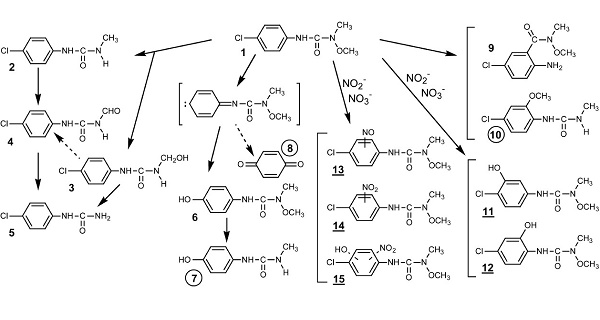
| [References]
[1] S. Nélieu. “Nitrite and nitrate induced photodegradation of monolinuron in aqueous solution.” Environmental Chemistry Letters 2 2 (2004): 83–87.
|
|
|