| Identification | Back Directory | [Name]
nicotinic anhydride | [CAS]
16837-38-0 | [Synonyms]
Ai3-15770
Einecs 240-860-6
nicotinic anhydride
Bisnicotinic anhydride
3-Pyridinecarboxylic Anhydride
3-PyridinecarboxylicAnhydride>
3-Pyridinecarboxylic acid, anhydride
nicotinic anhydride ISO 9001:2015 REACH
Bis(pyridine-3-carboxylic acid)anhydride
3-Pyridinecarboxylicacid, 1,1'-anhydride
3-PYRIDINECARBOXYLIC ANHYDRIDE , 97.0+%(GC)(T)
N-[(5-oxo-1,3-diphenyl-1,3,5$l^{5}-diazaphosphorinan-5-yl)methyl]aniline | [EINECS(EC#)]
240-860-6 | [Molecular Formula]
C12H8N2O3 | [MDL Number]
MFCD00055400 | [MOL File]
16837-38-0.mol | [Molecular Weight]
228.204 |
| Chemical Properties | Back Directory | [Melting point ]
124 °C | [Boiling point ]
200 °C / 1mmHg | [density ]
1.317±0.06 g/cm3(Predicted) | [solubility ]
soluble in Acetone | [form ]
powder to crystal | [pka]
2.51±0.10(Predicted) | [color ]
White to Almost white | [InChI]
InChI=1S/C12H8N2O3/c15-11(9-3-1-5-13-7-9)17-12(16)10-4-2-6-14-8-10/h1-8H | [InChIKey]
VPODXHOUBDCEHN-UHFFFAOYSA-N | [SMILES]
C1(C=NC=CC=1)C(=O)OC(=O)C1C=NC=CC=1 |
| Questions And Answer(Q&A) | Back Directory | [Preparation]
CAUTION: This reaction must be carried out in a hood and all precautions should be taken in the use of phosgene.
To a three-necked, 500 ml, round-bottomed flask equipped with a mechanical stirrer, pressure-equalized dropping funnel, distillation column and head, are added 10.0 gm (0.081 mole) of nicotinic acid and 275 ml of dry benzene. The mixture is heated and 75 ml of benzene is distilled in order to remove traces of moisture. The distillation column and head are removed and replaced by a thermometer and a calcium chloride drying tube. The reaction stirred mixture is cooled to 5°C in an ice bath, 8.65 gm (0.086 mole) of triethylamine is added all at once, and then 34 gm of a 12.5% solution of phosgene (0.043 mole) in benzene (available from Matheson, Coleman and Bell) is added slowly in order to keep the temperature below 7°C.
The reaction mixture is stirred for ¾ hr at room temperature, heated to boiling, and filtered hot using suction. The precipitated triethylamine hydrochloride is washed with three 25 ml positions of warm benzene and the resulting triethylamine amounts to 10.8 gm (96%). The combined benzene layer and washings are concentrated to dryness under reduced pressure at low temperatures. The solid residue is warmed with 75 ml of dry benzene, filtered hot, the remaining solid washed with two 5 ml portions of cold benzene, and the benzene filtrate and washes allowed to stand at 20°C for 2 - 3 hr. The anhydride crystallizes out of the benzene and is filtered. The solid is washed with two 4 ml portions of cold dry benzene and dried under reduced pressure to afford 6.25 gm (68%), m.p. 122-125°C. an additional 2.4 gm (25%) of product, m.p. 122- 123°C is obtained from the combined benzene filtrate and washes.
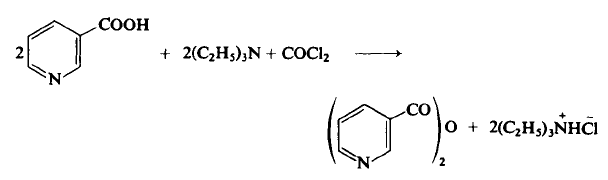
|
| Hazard Information | Back Directory | [Uses]
Nicotinic Anhydride is useful reactant for the synthesis of hydroxyl-?functionalized iron(II) bis(NHC) complexes. | [Synthesis Reference(s)]
Journal of the American Chemical Society, 69, p. 2231, 1947 DOI: 10.1021/ja01201a502 |
|
|