| Identification | Back Directory | [Name]
2-(tert-butyldiMethylsilyl )oxyl alcohol trifluorin Methanesulfonate | [CAS]
164162-36-1 | [Synonyms]
Everolimus Impurity 12
2-(t-ButyldiMethylsilyl)oxyethyl triflate
2-(t-Butyldimethylsilyloxy)EthylTrifluoromethanesulfonate
2-(tert-butyldiMethylsilyloxy)ethyl trifluoroMethanesulfonate
2-(tert-butyldiMethylsilyl )oxyl alcohol trifluorin Methanesulfonate
2-{[Dimethyl(2-methyl-2-propanyl)silyl]oxy}ethyl trifluoromethanesulfonate
Trifluoromethanesulfonic acid 2-[[(tert-butyl)dimethylsilyl]oxy]ethyl ester
Methanesulfonicacid,1,1,1-trifluoro-,2-[[(1,1-diMethylethyl)diMethylsilyl]oxy]ethylester
1,1,1-Trifluoromethanesulfonic acid 2-[[(1,1-dimethylethyl)dimethylsilyl]oxy]ethyl ester | [Molecular Formula]
C9H19F3O4SSi | [MDL Number]
MFCD22572946 | [MOL File]
164162-36-1.mol | [Molecular Weight]
308.39 |
| Hazard Information | Back Directory | [Uses]
2-(tert-butyldiMethylsilyl )oxyl alcohol trifluorin Methanesulfonate is used as organic synthesis intermediate and pharmaceutical intermediate, mainly used in laboratory research and development process and chemical production process. | [Synthesis]
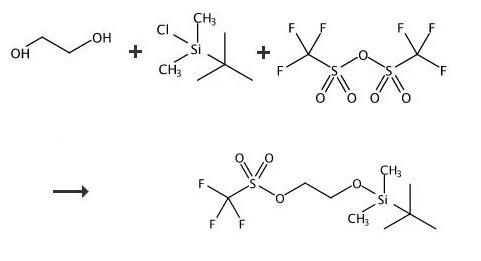
The reaction was carried out under a nitrogen atmosphere. TBS- ethylene glycol prepared as per step 1 (85.10g, 0.48 mol) and 2, 6-Lutidine (84.28ml, 0.72 mol) were stirred in n-heptane (425ml) to give a clear solution which was then cooled to -15 to - 25°C. Trifluoromethanesulfonic anhydride (Tf2O) (99.74 ml, 0.590 mol) was added drop-wise over a period of 45 minutes to the n-heptane solution (white precipitate starts to form immediately) while maintaining the reaction at -15 to -25°C. The reaction mixture was kept at temperature between -15 to -25°C for 2 hours. The precipitate generated was filtered off. The filtrate was then evaporated up to ~2 volumes with respect to TBS-ethyiene glycol (~200 ml). |
|
| Company Name: |
JSK Chemicals
|
| Tel: |
+919879767970 |
| Website: |
www.jskchemicals.com |
|