| Identification | Back Directory | [Name]
2'-O-(2-Methoxyethyl)-5-methyluridine | [CAS]
163759-49-7 | [Synonyms]
2'-O-MOE-Tr
5-Me-2'-O-MOE U
5-Me-2'-O-MOE Uridine
2'-O-MOE-5-Methyluridin
2'-O-Methoxyethyl-thyminde
2'-O-(2-Methoxyethy)-5-methyluridine
2'-O-(2-Methoxyethyl)-5-methyluridine
Uridine, 2'-O-(2-Methoxyethyl)-5-Methyl-
2'-O-(2-Methoxyethyl)-5-Methyl-uridine, >97%
1-[4-HYDROXY-5-(HYDROXYMETHYL)-3-(2-METHOXYETHOXY)OXOLAN-2-YL]-5-METHYL-3H-PYRIMIDINE-2,4-DIONE
1-[(2R,3R,4R,5R)-4-hydroxy-5-(hydroxymethyl)-3-(2-methoxyethoxy)oxolan-2-yl]-5-methylpyrimidine-2,4-dione | [EINECS(EC#)]
500-100-4 | [Molecular Formula]
C13H20N2O7 | [MDL Number]
MFCD02682964 | [MOL File]
163759-49-7.mol | [Molecular Weight]
316.31 |
| Chemical Properties | Back Directory | [Melting point ]
115.5-116.5 °C | [density ]
1.41 | [storage temp. ]
Sealed in dry,2-8°C | [solubility ]
DMSO (Slightly), Methanol (Slightly), Water (Slightly, Sonicated) | [form ]
Solid | [pka]
9.55±0.10(Predicted) | [color ]
Off-White | [Stability:]
Hygroscopic | [InChI]
InChI=1S/C13H20N2O7/c1-7-5-15(13(19)14-11(7)18)12-10(21-4-3-20-2)9(17)8(6-16)22-12/h5,8-10,12,16-17H,3-4,6H2,1-2H3,(H,14,18,19)/t8-,9-,10-,12-/m1/s1 | [InChIKey]
NEVQCHBUJFYGQO-DNRKLUKYSA-N | [SMILES]
OC[C@H]1O[C@@H](N2C=C(C)C(=O)NC2=O)[C@H](OCCOC)[C@@H]1O |
| Hazard Information | Back Directory | [Uses]
2'-O-(2-Methoxyethyl)-5-methyluridine is a protected uridine derivative (U829910) and nucleoside used in the preparation of synthetic nucleic acids. | [Application]
2'-O-(2-Methoxyethyl)-5-methyluridine (2'-MOE-5-Methyluridine) is a uridine nucleoside that is a combination of amidite with 5-methyluridine. 2'-MOE-5-Methyluridine is an intermediate in the synthesis of oligonucleotides, which are synthesized from phosphoramidites. Its analog 5'-O-DMT-2'-O-(2-methoxyethyl)-5-methyluridine is a potent compound used in research of RNA viruses, particularly hepatitis C virus and picornaviruses. | [Synthesis]
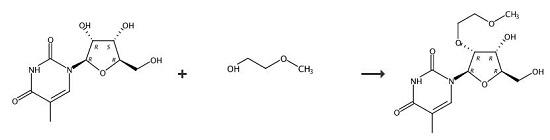
2,2'-Anhydro-5-methyluridine (195 g, 0.81 M), tris(2-methoxyethyl)borate (231 g, 0.98 M) and 2-methoxyethanol (1.2 L) were added to a 2 L stainless steel pressure vessel and placed in a pre-heated oil bath at 160°C. After heating for 48 hours at 155-160°C, the vessel was opened and the solution evaporated to dryness and triturated with methanol (200 mL). The residue was suspended in hot acetone (1 L). The insoluble salts were filtered, washed with acetone (150 mL) and the filtrate evaporated. The residue (280 g) was dissolved in CH3CN (600 mL) and evaporated. A silica gel column (3 kg) was packed in CH2Cl2/ acetone/methanol (20:5:3) containing 0.5% Et3NH. The residue was dissolved in CH2Cl2 (250 mL) and adsorbed onto silica (150 g) prior to loading onto the column. The product was eluted with the packing solvent to give product. Yield 160 g, 63%. |
|
|