| Identification | Back Directory | [Name]
cangrelor tetrasodium | [CAS]
163706-36-3 | [Synonyms]
CS-1027
angrelor tetrasodium
cangrelor tetrasodium
Cangrelor Tetrasodium Salt
AR-C 69931 Tetrasodium Salt
Cangrelor tetrasodium salt >=98% (HPLC)
tetrasodium 5'-O-[({[dichloro(phosphonato)methyl]phosphinato...
N-[2-(Methylthio)ethyl]-2-[(3,3,3-trifluoropropyl)thio]adenosine-5'-O-(β,γ-dichloromethylene)triphosphate tetrasodium salt
tetrasodium 5'-O-[({[dichloro(phosphonato)methyl]phosphinato}oxy)phosphinato]-N-[2-(methylsulfanyl)ethyl]-2-[(3,3,3-trifluoropropyl)sulfanyl]adenosine
(Dichloro((((((2R,3S,4R,5R)-3,4-dihydroxy-5-(6-((2-(methylthio)ethyl)amino)-2-((3,3,3-trifluoropropyl)thio)-9H-purin-9-yl)tetrahydrofuran-2-yl)methoxy)(hydroxy)phosphoryl)oxy)(hydroxy)phosphoryl)methyl)phosphonic acid tetrasodium salt | [EINECS(EC#)]
813-784-1 | [Molecular Formula]
C17H21Cl2F3N5Na4O12P3S2 | [MDL Number]
MFCD14635359 | [MOL File]
163706-36-3.mol | [Molecular Weight]
864.287 |
| Chemical Properties | Back Directory | [storage temp. ]
4°C, away from moisture and light | [solubility ]
DMSO:100.0(Max Conc. mg/mL);115.7(Max Conc. mM) | [form ]
A solid | [color ]
White to off-white | [Water Solubility ]
Soluble to 100 mM in water |
| Hazard Information | Back Directory | [Description]
Cangrelor
tetrasodium is a direct purinergic platelet receptor (P2Y12)
inhibitor that blocks ADP-induced platelet activation and
aggregation. The drug, which was developed by The
Medicine Company, binds reversibly to the P2Y12 receptor,
preventing further signaling and platelet activation. Cangrelor,
which was approved in June 2015 by the FDA, is indicated as
an adjunct to percutaneous coronary intervention for reducing
the risk of periprocedural myocardial infarction, repeat
coronary revascularization, and stent thrombosis in patients
who have not been treated with a P2Y12 platelet inhibitor and
are not being given a glycoprotein IIb/IIIa inhibitor. The
most common side effect observed with the drug was
bleeding. | [Uses]
Antiplatelet agent. | [Definition]
ChEBI: An organic sodium salt that is the tetrasodium salt of cangrelor. Used as an intravenous antiplatelet drug that prevents formation of harmful blood clots in the coronary arteries. | [Synthesis]
While several discovery-scale routes to cangrelor tetrasodium
were previously reported,14 an improved procedure developed
with the goal of providing a manufacturing scale route to
cangrelor tetrasodium has recently been reported by Jinan
Bestcomm Pharmaceutical R&D. Starting from commercially
available 2-thiobarbituric acid (36), S-alkylation
with 3,3,3-trifluoropropyl iodide proceeded in high yield (94%)
under basic conditions. Nitration of this intermediate with
HNO3/AcOH generated a nitro-pyrimidine diol in 80% yield.
Bis-chlorination via treatment with POCl3
provided the
corresponding dichloro pyrimidine (92%), and subsequent
nitro reduction with AcOH/Fe under aqueous conditions
yielded intermediate 37 (quantitative yield), which readily
provided the bis-aniline analogue by reaction with ammonia in
EtOH/H2O at 80 ??C. Condensation with triethyl orthoformate/
HCl at room temperature provided access to the desired
purine in high yield (97%). A one-pot alkylation/amination
strategy was then employed, first relying on S-alkylation of 2-
aminoethanethiol hydrochloride with MeI/NaOH and reaction
of the resulting amine with the purine chloride generated 38 in
88% across the sequence. Alkylation of 38 with commercial
furanose 39 proceeded in a regioselective manner favoring N-9
functionalization, employing conditions similar to those
previously described by Almond and co-workers. Toward
this end, silylation of 38 with N,O-bis-(trimethylsilyl)acetamide
(BSA) followed by subjection to TMSOTf and 39, resulted in
the desired N-9 alkylated product, which was carried crude to
global deacetylation with NaOH/EtOH at room temperature,
making way for smooth conversion to alcohol 40 (87% from
38). Although phosphorylation of 40 has been performed using
a variety of related methods,14 the largest scale conditions
reported to date consist of initial 5?? alcohol activation with
POCl3 and PO(OEt)3 in the presence of 1,8-diaminonaphthalene,
furnishing the 5?? monophosphonate intermediate. This
intermediate was not isolated but further treated with a solution
of dichloromethylenebis(phosphonic acid) tributylammonium
salt and tributylamine in DMF at 0 ??C, yielding cangrelor as a
crude ammonium salt following quench with NH4HCO3.15
Purification via ion exchange chromatography provided
cangrelor as its ammonium salt in 68% yield over the threestep
sequence, which was subjected to aqueous NaHCO3
solution and lyophilization and provided cangrelor tetrasodium
salt (IV). This synthesis was performed starting on >100 g scale
of 36 and required only one chromatography step which
involved ion exchange chromatography of the cangrelor
ammonium salt. More recently, while beyond the scope of
this article, additional reports have been disclosed describing
the development of specific pharmaceutical formulations for
delivery of cangrelor in high purity.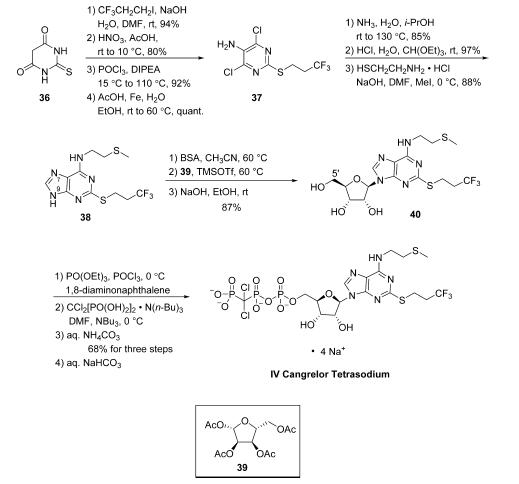
| [storage]
Store at -20°C |
|
|