| Identification | Back Directory | [Name]
Dibenzo[b,d]furan-1-ylboronic acid | [CAS]
162607-19-4 | [Synonyms]
Dibenzofuran-1-boronic acid
1-Dibenzofuranylboronic acid
B-1-dibenzofuranylBoronic acid
Dibenzo[b,d]furan-1-boronic acid
Boronic acid, B-1-dibenzofuranyl-
Dibenzo[b,d]furan-1-ylboronic acid
1-Dibenzofuranylboronic Acid ISO 9001:2015 REACH
Dibenzofuran-1-boronic Acid (contains varying amounts of Anhydride) | [EINECS(EC#)]
829-640-6 | [Molecular Formula]
C12H9BO3 | [MDL Number]
MFCD01318978 | [MOL File]
162607-19-4.mol | [Molecular Weight]
212.009 |
| Chemical Properties | Back Directory | [Boiling point ]
438.5±37.0 °C(Predicted) | [density ]
1.34±0.1 g/cm3(Predicted) | [storage temp. ]
Inert atmosphere,2-8°C | [form ]
powder to crystal | [pka]
8.20±0.30(Predicted) | [color ]
White to Almost white |
| Questions And Answer | Back Directory | [Uses]
1-Dibenzofuranylboronic Acid is the reagent for electronic devices materials preparation. There are some synthesis routes to describe how do 1-dibenzofuranylboronic Acid works as the reagents for organic light emitting diode (OLED) applications.
1-Dibenzofuranylboronic acid works as intermediate to synthesis the dibenzo [g, p] chrysene compound.
|
| Hazard Information | Back Directory | [Synthesis]
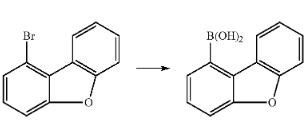
180 g (728 mmol) of 1-bromodibenzofuran aredissolved in 1500 ml of dry THF and cooled to -78?? C. 305 ml (764 mmol/2.5 M in hexane) of n-butyllithium are added at this temperature over the course of about 5 min., and the mixture is subsequently stirred at -78?? C. for a further 2.5 h. 151 g (1456 mmol) of trimethyl borate are added as rapidly as possible at this temperature, and the reaction mixture is allowed to come slowly to room temperature (about 18 h). The reaction solution is washed with water, and the precipitated solid and the organic phase are dried azeo- tropically with toluene. The crude product is washed by stirring with toluene/methylene chloride at about 40?? C. and filtered off with suction. Yield: 146 g (690 mmol), 95% of theory. |
|
|