| Identification | Back Directory | [Name]
N-(PHENYLTHIO)PHTHALIMIDE | [CAS]
14204-27-4 | [Synonyms]
N-(PHENYLTHIO)PHTHALIMIDE
N-(Phenylthio)phthalimide
2-(Phenylthio)-1,3-isoindolinedione
2-(Phenylthio)isoindoline-1,3-dione | [Molecular Formula]
C14H9NO2S | [MDL Number]
MFCD00192396 | [MOL File]
14204-27-4.mol | [Molecular Weight]
255.29 |
| Questions And Answer(Q&A) | Back Directory | [Preparation]
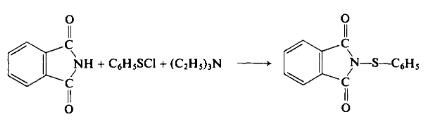
(a) Preparation of benzenesulfenyl chloride. To a stirred solution of 55 gm (0.5 mole) of benzene thiol in 300 ml of η -pentane at 0°C is added chlorine gas until an assay (GC) of the resulting red-orange solution shows quantitative conversion to the sulfenyl chloride. The reaction usually requires about 39 gm (0.6 mole) of chlorine.
(b) Reaction of benzenesulfenyl chloride with phthalimide. To a stirred solution of 73.5 gm (0.5 mole) of phthalimide in 200 ml of dimethylforma-mide is first added 60 gm (0.6 mole) of triethylamine. Then the sulfenyl chloride solution from (a) is slowly added dropwise. The reaction mixture is stirred for \ hr, poured into 2 liters of cold water, filtered, and dried to afford 121 gm (95%), m.p. 160-161°C (recrystallized from ethanol).
Complex imides such as camphorimide and 9,10-dihydroanthracene-9,10-endo-a,/3-succinimide have been alkylated in methylene dichloride with alkyl halides, using tetrabutylammonium bromide as a phase-transfer catalyst and aqueous potassium hydroxide as a base.
|
|
| Company Name: |
Energy Chemical
|
| Tel: |
021-021-58432009 400-005-6266 |
| Website: |
http://www.energy-chemical.com |
|