| Identification | Back Directory | [Name]
2-Amino-4-bromo-3-fluorobenzoic acid | [CAS]
1416013-62-1 | [Synonyms]
140622
3-BROMO-6-CARBOXY-2-FLUOROANILINE
2-Amino-4-bromo-3-fluorobenzoic acid
Benzoic acid, 2-amino-4-bromo-3-fluoro- | [Molecular Formula]
C7H5BrFNO2 | [MDL Number]
MFCD27664889 | [MOL File]
1416013-62-1.mol | [Molecular Weight]
234.023 |
| Chemical Properties | Back Directory | [Boiling point ]
332.0±42.0 °C(Predicted) | [density ]
1.877±0.06 g/cm3(Predicted) | [storage temp. ]
Keep in dark place,Inert atmosphere,2-8°C | [form ]
powder | [pka]
4.37±0.10(Predicted) | [color ]
Off white | [InChI]
InChI=1S/C7H5BrFNO2/c8-4-2-1-3(7(11)12)6(10)5(4)9/h1-2H,10H2,(H,11,12) | [InChIKey]
SFRJQFICUQFDFU-UHFFFAOYSA-N | [SMILES]
C(O)(=O)C1=CC=C(Br)C(F)=C1N |
| Hazard Information | Back Directory | [Description]
2-Amino-4-bromo-3-fluorobenzoic acid is an important pharmaceutical and organic intermediate ingredient used in the synthesis of avibactam sodium, an FDA-approved non-beta-lactam β-lactamase inhibitor used in combination with ceftazidime (Avycaz). It is used for the treatment of complicated intra-abdominal infections, urinary tract infections, and pyelonephritis. | [Uses]
2-Amino-4-Bromo-3-Fluorobenzoic Acid is used in preparation of Quinazoline compounds as KRAS modulators and antitumor agents. | [Synthesis]
The synthesis of 2-Amino-4-bromo-3-fluorobenzoic acid is as follows:
To a mixture of 6-bromo-7-fluoroindoline-2,3-dione (18.9 g, 77.5 mmol) in 2 N NaOH (350 mL) at 0 oC, H2O2 (30%, 40 mL) was added. The mixture was stirred at room temperature for 16 h. The mixture was quenched with Na2SO3, and the mixture was acidified with conc. HCl to adjust pH to 2. The precipitate was collected by filtration and dried to afford the desired product as a white solid (17 g, 94% yield).
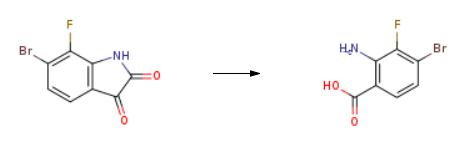 |
|
|