| Identification | Back Directory | [Name]
(R,R)-(-)-N,N'-BIS(3,5-DI-TERT-BUTYLSALICYLIDENE)-1,2-CYCLOHEXANEDIAMINO-MANGANESE(III) CHLORIDE | [CAS]
138124-32-0 | [Synonyms]
Jacobsen
96+%(chn)
(R,R)-form
(R,R)Jacobesn
JACOBSEN'S CATALYST
(R,R)-JACOBSEN CATALYST
Jacobsen'scatalyst(R,R)
(R,R)-JACOBSEN'S CATALYST
-1,2- cyclohexanediaminomanganese(III)
-N,N'-Bis(3,5-di-tertbutylsalicylidene)
(-)-BIS(3,5-DI-T-BU-SALICYL.)-1,2-CYCLO-
(R,R)-Jacobsen’s catalyst, Jacobsen’s catalyst
(R,R)-JACOBSEN'S CATALYST MANGANESE(III) CHLORIDE COMPLEX
(R,R)-N,N -BIS(3,5-DI-TERT-BUTYLSALICYL- IDENE)-1,2-CYCLOHEXANEDIAMINO-MN-CL
(R,R)-N,N'-BIS(3,5-DI-TERT-BUTYLSALICYL- IDENE)-1,2-CYCLOHEXANEDIAMINO-MN-CL98%
(R,R)(-)N,N'Bis(3,5-di-tbutylsalicylidene)1,2-chexanediaminomanganese(iii)chlorideco
(R,R)-(-)N,N'-Bis(3,5-di-tert-butylsalicylidene)-1,2-cyclohexanediaminomanganese(III) chlo
(R,R)-(-)-N,N'-BIS(3.5-DI-T-BUTYLSALICYLIDENE)-1,2-CYCLOHEXANEDIAMINE-MANGANESE (III) CHLORIDE
(R,R)-(-)-N,N'-Bis(3,5-di-tert-butylsalicylidene)-1,2-cyclohexanediaminemanganese(III)chloride
(1R,2R)-(-)-[1,2-CYCLOHEXANEDIAMINO-N N'-BIS(3,5-DI-T-BUTYLSALICYLIDENE)]MANGANESE(III)CHLORIDE
(R,R)-(-)-N,N'-BIS(3,5-DI-TERT-BUTYLSALICYLIDENE)-1,2-CYCLOHEXANEDIAMINO-MANGANESE(III) CHLORIDE
(R,R)-(-)-[N,N(1)-bis(3,5-di-tert-butylsalicylidene)-1,2-cyclohexanediaMinoat(2-)]Manganese(III) chloride
(1R,2R)-(-)-[1,2-CyclohexanediaMino-N,N'-bis(3,5-di-t-butylsalicylidene)]Manganese (III) chloride (R,R)-Jacobsen Cat.
(1R,2R)-(-)-[1,2-Cyclohexanediamino-N,N'-bis(3,5-di-t-butylsalicylidene)]manganese(III)chloride,98%(R,R)-JacobsenCat.
Manganese,chloro[[2,2'-[(1R,2R)-1,2-cyclohexanediylbis[(nitrilo-kN)methylidyne]]bis[4,6-bis(1,1-dimethylethyl)phenolato-kO]](2-)]-, (SP-5-13)- | [Molecular Formula]
C36H52ClMnN2O2 | [MDL Number]
MFCD02101664 | [MOL File]
138124-32-0.mol | [Molecular Weight]
635.2 |
| Chemical Properties | Back Directory | [Appearance]
Dark brown powder or chunks | [Melting point ]
330-332 °C(lit.)
| [alpha ]
D23 +580° (c = 0.01 in ethanol) | [storage temp. ]
Inert atmosphere,2-8°C | [form ]
Liquid May Develop Some Turbidity or Precipitate | [color ]
Light yellow to gold to brown | [λmax]
509nm(CH2Cl2)(lit.) | [Merck ]
14,5252 | [InChIKey]
LJVAWOSDJSQANR-OHRASPNLSA-K |
| Hazard Information | Back Directory | [Chemical Properties]
Dark brown powder or chunks | [Uses]
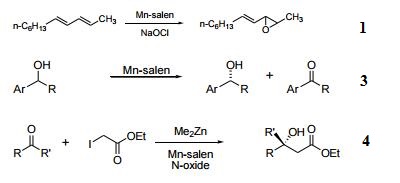
(R,R)-[N,N'-Bis(3,5-di-tert-butylsalicylidene)-1,2-cyclohexanediamine]manganese(III) Chloride is a coordinated compound of manganese and a salen-type ligand. It is also used as an asymmetric catalyst
used to enantioselectively transform prochiral alkenes into epoxides. | [Uses]
Chiral catalyst for epoxidation of olefins. | [General Description]
We are committed to bringing you Greener Alternative Products, which adhere to one or more of The 12 Principles of Greener Chemistry. This product has been enhanced for catalytic efficiency. Click here for more information. |
| Questions and Answers (Q&A) | Back Directory | [Reactions]
- Catalyst for the conversion of olefins to chiral epoxides in high enantiomeric excess.
- Pharmaceutically important, single enantiomer amino alcohols are efficiently produced from the corresponding chiral epoxide by acid or base-catalyzed epoxide ring-opening reactions.
- symmetric Kinetic resolution of secondary alcohols in water.
- Enantioselective Reformatsky reaction with ketones.
|
|
|