| Identification | Back Directory | [Name]
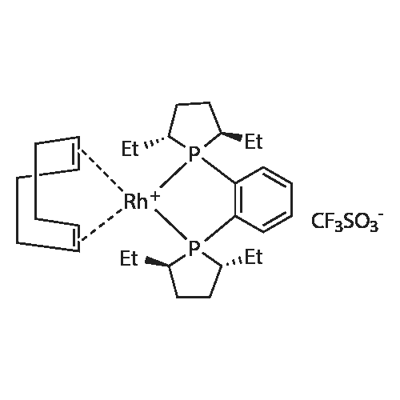
(-)-1,2-BIS((2R,5R)-2,5-DIETHYLPHOSPHOLANO)BENZENE(CYCLOOCTADIENE)RHODIUM(I)TRIFLUOROMETHANESULFONATE | [CAS]
136705-77-6 | [Synonyms]
(R,R)-ET-DUPHOS-RH
Bisdiethylphospholanobenzenecyclooctadienerhodiumtrifluorometanesulfon
(-)-1,2-BIS-((2R,5R)DIETHYLPHOSPHOLANO)BENZENE(CYCLOOCTADIENE)RHODIUM(I) TRIFLATE
(-)-1,2-Bis[(2R,5R)-diethylphospholano)benzene(cyclooctadiene]rhodium(I) triflate,98%
(-)-1,2-Bis((2R,5R)-2,5-diethylphospholano)benzene(cyclooctadiene)rhodium(I) trifluoromethanesulfona
(+)-1,2-Bis((2S,5S)-2,5-diethylphospholano)benzene(cyclooctadiene)rhodium(I) trifluoromethansulfonate
(-)-1,2-BIS(2R,5R)-2,5-(DIETHYLPHOSPHOLONO)BENZENE(CYCLOACTADIENE)RHODIUM(I)TRIFLUOROMETHANESULFONATE
(-)-1,2-BIS((2R,5R)-2,5-DIETHYLPHOSPHOLANO)BENZENE(CYCLOOCTADIENE)RHODIUM(I)TRIFLUOROMETHANESULFONATE
1,2-Bis[(2R,5R)-2,5-diethylphospholano]benzene(1,5-cyclooctadiene)rhodium(I) trifluoromethanesulfonate
(-)-1,2-Bis((2R,5R)-2,5-diethylphospholano)benzene(cyclooctadiene)rhodium(I)trifluoromethanesulfonate(r,r)-et-duphos-rh
(-)-1,2-Bis((2R,5R)-2,5-diethylphospholano)benzene(cyclooctadiene)rhodium(I)trifluoromethanesulfonate,98+%(R,R)-Et-DUPHOS-Rh
(-)-1,2-Bis((2R,5R)-2,5-diethylphospholano)benzene(1,5-cyclooctadiene)rhodiuM(I) trifluoroMethanesulfonate (R,R)-Et-DUPHOS-Rh
(-)-1,2-Bis((2R,5R)-2,5-diethylphospholano)benzene(1,5-cyclooctadiene)rhodium(I) trifluoromethanesulfonate, 98+% (R,R)-Et-DUPHOS-Rh | [Molecular Formula]
C31H48F3O3P2RhS | [MDL Number]
MFCD00269860 | [MOL File]
136705-77-6.mol | [Molecular Weight]
722.62 |
| Chemical Properties | Back Directory | [Appearance]
orange to orange-brown crystals | [storage temp. ]
Refrigerator (+4°C) | [form ]
crystal | [color ]
orange | [Sensitive ]
Air Sensitive | [Exposure limits]
ACGIH: TWA 1 mg/m3
NIOSH: IDLH 100 mg/m3; TWA 0.1 mg/m3 |
| Hazard Information | Back Directory | [Chemical Properties]
orange to orange-brown crystals | [Uses]
DuPhos and BPE Ligands: Highly Efficient Privileged Ligands
Catalyst for:
- Stereoselective synthesis of δ-amino acid derivatives via asymmetric hydrogenation of acetylaminopentenoic acid derivatives
- Stereoselective synthesis of manzacidins A and C via stereoselective hydrogenation
- Stereoselective synthesis of tetracyclic core of manzamine A via Rh-catalyzed asymmetric hydrogenation, diastereoselective Diels-Alder reaction, Eschenmoser-Tanabe fragmentation, Chang′s amide formation, and Hofmann rearrangement
- Asymmetric preparation of chiral Cbz-aminodifluorobutyric acid Me ester and its analogs
- Biphasic catalytic hydrogenations in ionic liquids with addition of water as a second solvent
- Preparation of cyclobutane-containing amino acids via asymmetric hydrogenations of cyclobutyl enamides
- Asymmetric preparation of both enantiomers of (dimethoxycoumaryl)alanine as suitable fluorescent peptide labels
|
| Questions And Answer | Back Directory | [Reaction]
- The DUPHOS family of catalysts is highly efficient for the asymmetric hydrogenation of various substituted acetamidoacrylates and enol acetates yielding products of high enantiomeric excesses. Efficient ligand for the asymmetric hydrogenation of tetrasubstituted enamides.
- Forms superior catalysts for asymmetric reductive aminations.
- Catalyst used for the asymmetric hydrogenation of enol phosphonates.
- A novel enantioselective synthesis of β-amino alcohols and 1,2-diamines.
- Ligand for the catalytic asymmetric [4+1] cycloaddition of vinylallenes with CO.
- Ligand for the Rh-catalyzed asymmetric enyne cycloisomerization.
- Catalytic enantioselective addition of dialkylzinc to N-Diphenylphosphinoylimines.
- Palladium catalyzed asymmetric phosphination.

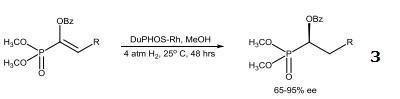


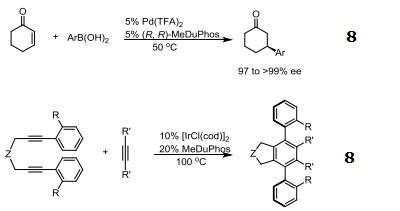
|
|
| Company Name: |
Alfa Aesar
|
| Tel: |
400-6106006 |
| Website: |
http://chemicals.thermofisher.cn |
| Company Name: |
Energy Chemical
|
| Tel: |
021-021-58432009 400-005-6266 |
| Website: |
http://www.energy-chemical.com |
| Company Name: |
Sigma-Aldrich
|
| Tel: |
021-61415566 800-8193336 |
| Website: |
https://www.sigmaaldrich.cn |
| Company Name: |
Alfa Chemistry
|
| Tel: |
1-516-6625404 |
| Website: |
https://www.alfa-chemistry.com |
|