| Identification | Back Directory | [Name]
5-ACETYL-1H-PYRAZOLE-3-CARBOXYLIC ACID | [CAS]
1297537-45-1 | [Synonyms]
ODM-201 int1
ODM-201 intermediate 1
5-Acetylpyrazole-3-carboxylic Acid
5-ACETYL-1H-PYRAZOLE-3-CARBOXYLIC ACID
1H-Pyrazole-3-carboxylic acid, 5-acetyl-
5-ACETYL-1H-PYRAZOLE-3-CARBOXYLIC ACID(For export only) | [Molecular Formula]
C6H6N2O3 | [MDL Number]
MFCD09028402 | [MOL File]
1297537-45-1.mol | [Molecular Weight]
154.12 |
| Chemical Properties | Back Directory | [Melting point ]
257-269 °C (decomp)(Solv: ethanol (64-17-5); benzene (71-43-2)) | [Boiling point ]
479.2±30.0 °C(Predicted) | [density ]
1.467±0.06 g/cm3(Predicted) | [storage temp. ]
Sealed in dry,2-8°C | [pka]
3.60±0.10(Predicted) | [InChI]
InChI=1S/C6H6N2O3/c1-3(9)4-2-5(6(10)11)8-7-4/h2H,1H3,(H,7,8)(H,10,11) | [InChIKey]
HFBWRCZRDIVAMQ-UHFFFAOYSA-N | [SMILES]
N1C(C(C)=O)=CC(C(O)=O)=N1 |
| Hazard Information | Back Directory | [Uses]
5-acetyl-1H-pyrazole-3-carboxylic acid can be used in the industrialization of substances such as polyurethanes. Impurities in this chemical are removed through filtration before it is used for synthesis. | [Definition]
5-Acetyl-1H-pyrazole-3-carboxylic acid can be considered a valuable building block for other types of organic and medicinal chemistry transformations and is the essential starting material for the preparation of Darolutamide, which is chemically known as N-{(2S)-1-[3-(3-chloro-4- cyanophenyl)-1H-pyrazol-1-yl]propan-2-yl}-5-(1-hydroxyethyl)-1H-pyrazole-3- 10 carboxamide. Orion Corporation and Bayer Health Care developed Darolutamide. It is an anti-androgen medication that is used for the treatment of prostate cancer in men. Recently, a safe and metal-free process using ethyl glycinate hydrochloride as the starting material has been developed to prepare 5-Acetyl-1H-Pyrazole-3-carboxylic acid[1-2].
| [Synthesis]
5-Acetyl-1H-pyrazole-3-carboxylic acid is a chemical compound that is synthesized from hydrazine and acetyl chloride.
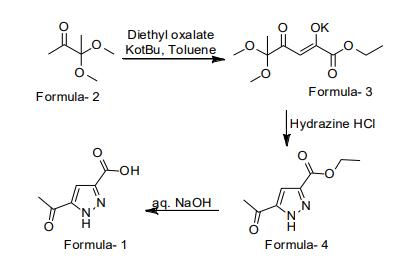
Potassium tert-butoxide (102 g) was added lot-wise to the pre-cooled mixture of 3,3-dimethoxybutane-2-one (100 g) in toluene (1000 ml) at 5-10°C and stirred for 45 minutes. Diethyl oxalate (132 g) was added to the mixture at 10-15°C and stirred for 4 hours. Filtered the solid. To the filtered solid water (3000 ml) was added and cooled to 5-10°C. Solution of Hydrazine monohydrochloride (51.8 g) was slowly added to the mixture at 5-10°C and stirred for 4 hrs. Dichloromethane (1000 ml) was added to the mixture at 5-10°C and stirred for 15 minutes. Allowed to heat the mixture to 25-30°C and stirred for 15 minutes. Filtered the mixture through hyflow. Organic layer was separated from the filtrate. Distilled-off the organic layer and co-distilled in n-heptane (50 ml). n-heptane (300 ml) was added to the mixture at 25-30°C. Heated the mixture at 45-50°C and stirred for 1 hrs. Allowed to cool the mixture to 25-30°C and stirred for 3 hours. Filtered the solid. THF (150 ml) was added to the obtained solid. aq. Sodium hydroxide solution was added to the mixture at 10-15°C. Heated the mixture to 55-60°C and stirred for 2 hrs. Allowed to cool the mixture to 25-30°C and stirred for 15 minutes. Separated the aqueous layer from organic layer. Water was added to the aqueous layer at 25-30°C. Cooled the mixture to 20-25°C. Treated the mixture with hydrochloric acid at 20-25°C. Allowed to heat the mixture to 25-30°C and stirred for 4 hours. Filtered the solid and dried. Tetrahydrofuran (300 ml) was added to the obtained solid at 25-30°C. Heated the mixture to 50-55°C and stirred for 60 minutes. Allowed to cool the mixture to 25-30°C and stirred for 3 hours. Filtered the solid and dried to get 5-Acetyl-1H-pyrazole-3-carboxylic acid, Yield: 63 gm Purity by HPLC: 99.93%.
| [References]
[1] László Poszávácz. "New, Scalable Process for the Preparation of 5-Acetyl-1 H -pyrazole-3-carboxylic Acid, a Key Intermediate of Darolutamide." Synthesis-Stuttgart 33 2 (2023): 2061–2069.
[2] Bence Szilágyi. "Safe and Efficient Continuous-Flow Synthesis and Batchwise Hydrolysis of Ethyl 5-Acetyl-1 H -pyrazole-3-carboxylate: A Key Synthon of Darolutamide." Synthesis-Stuttgart 212 1 (2022): 959–966.
|
|
|