| Identification | Back Directory | [Name]
Rockphos | [CAS]
1262046-34-3 | [Synonyms]
Rockphos
RockPhos 97%
2-Di-tert-butylphosphino-3-Methoxy-6-Methyl-2'-4'-6'-triisopropylbiphenyl
2-Di(tert-butyl)phosphino-2′,4′,6′-triisopropyl-3-methoxy-6-methylbiphenyl
2-(Di-t-butylphosphino)-3-methoxy-6-methyl-2',4',6'-tri-i-propyl-1,1'-biphenyl
2-Di-tert-butylphosphino-3-Methoxy-6-Methyl-2',4',6'-triisopropyl-1,1'-biphenyl
2-Di-tert-butylphosphino-3-Methoxy-6-Methyl-2'-4'-6'-triisopropylbiphenyl, 98% Min
Di-tert-butyl(2′,4′,6′-triisopropyl-3-methoxy-6-methyl-[1,1′-biphenyl]-2-yl)phosphine
2-(Di-t-butylphosphino)-3-Methoxy-6-Methyl-2'-4'-6'-tri-i-propyl-1,1'-biphenyl, Min. 98% RockPhos
Bis(1,1-dimethylethyl)[3-methoxy-6-methyl-2',4',6'-tris(1-methylethyl)[1,1'-biphenyl]-2-yl]phosphine | [EINECS(EC#)]
805-149-2 | [Molecular Formula]
C31H49OP | [MDL Number]
MFCD20922895 | [MOL File]
1262046-34-3.mol | [Molecular Weight]
468.694 |
| Questions And Answer | Back Directory | [Reaction]
Ligand used in the palladium-catalyzed C-O bond forming reactions of secondary and primary alcohols with a range of aryl halides. Heterocyclic halides and, for the first time, electron-rich aryl halides can be coupled with secondary alcohols.
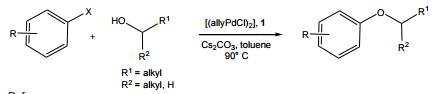
|
| Hazard Information | Back Directory | [Uses]
RockPhos is a biphenyl-based phosphine ligand used with Pd(0) catalyst for carbon-carbon and carbon-heteroatom bond formation reactions.
It can also be employed in the:
- 2-fluroethoxylation of bromo-chalcones in the presence of palladium catalyst.
- Conversion of aryl chlorides into aryl methoxides using RockPhos Pd G3 as a catalyst.
| [Uses]
Rockpahos is commonly used as a catalyst in organic reactions along with a palladium compound catalyst. | [reaction suitability]
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: ligand
reaction type: Cross Couplings |
|
|