| Identification | Back Directory | [Name]
ethyl-2,2-dimethyl-7-bromoheptanoate | [CAS]
123469-92-1 | [Synonyms]
ETC-1002 intermediate 2
Bempedoic Acid Impurity 9
Bempedoic acid intermediate
ethyl 7-bromo-2,2-dimethylheptanoate
ethyl-2,2-dimethyl-7-bromoheptanoate
7-Bromo-2,2-dimethylheptanoic acid ethyl ester
Heptanoic acid, 7-bromo-2,2-dimethyl-, ethyl ester | [Molecular Formula]
C11H21BrO2 | [MDL Number]
MFCD27942427 | [MOL File]
123469-92-1.mol | [Molecular Weight]
265.19 |
| Chemical Properties | Back Directory | [Boiling point ]
106-108 °C(Press: 0.01 Torr) | [density ]
1.169±0.06 g/cm3(Predicted) | [InChI]
InChI=1S/C11H21BrO2/c1-4-14-10(13)11(2,3)8-6-5-7-9-12/h4-9H2,1-3H3 | [InChIKey]
SYRIIFZUZOIRNU-UHFFFAOYSA-N | [SMILES]
C(OCC)(=O)C(C)(C)CCCCCBr |
| Hazard Information | Back Directory | [Description]
Ethyl 7-bromo-2,2-dimethylheptanoate is an important raw material in the synthesis route of bepetidic acid. Bempedoic acid, whose chemical name is 8-hydroxy-2,2,14,14-tetramethylpentadecanedioic acid, also known as ETC-1002, is a new type of lipid developed by the American EsperionTherapeutic company Quality regulation small molecule drugs. As one of the company's main drug candidates, its targets are liver adenosine triphosphate-citrate lyase (ACL) and adenosine monophosphate-activated protein kinase (AMPK). | [Uses]
Ethyl-2,2-dimethyl-7-bromoheptanoate can be used as an intermediate in organic synthesis, mainly used in laboratory research and development and chemical production processes. | [Synthesis]
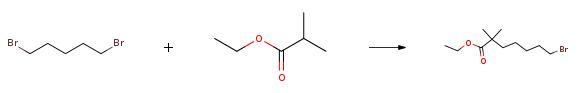
The specific embodiment of the present invention is as follows: prepare 19.4g (167mmol) of ethyl isobutyrate in 216mL solution of tetrahydrofuran with a mass concentration of 10%, connect to metering pump 1; prepare 84mL (168mmol) of 2.0M n-butyllithium solution, connect to meter Pump 2; configure 1,5-dibromopentane 80.0g (348mmol) tetrahydrofuran 100mL solution, mass concentration is 63%, connected to metering pump 3.Configure 300mL of 1N hydrochloric acid solution and connect to metering pump 4.Set continuous flow precooler 1, the circulating temperature is 0, and reach stability; set continuous flow precooler 2, the circulating temperature is 0, and reach stability; set continuous flow precooler 3, and the circulating temperature is 0, and reach stability; set continuous flow precooler 4, cycle temperature is 0, and reach stability;Set the flow rate of metering pump 1 to 65.0ml/min, set the flow rate of metering pump 2 to 25.0ml/min, set the flow rate of metering pump 3 to 30.0ml/min, and set the flow rate of metering pump 4 to 90.0ml/min.Turn on the metering pump 1 and metering pump 2 at the same time. After 60s of operation, turn on the metering pump 3, and after 60s of operation, turn on the metering pump 4 to collect the outflowing reaction liquid in the storage tank.After the operation is completed, separate the liquids, collect the upper organic phase, concentrate under reduced pressure at P=-0.08MPa, T=45??C to remove low-boiling solvents, and then rectify under reduced pressure at P=5mmHg and T=70??C to obtain 38.4 g.The obtained product is a colorless liquid with a GC purity of 98.6% and a yield of 87%. |
|
|