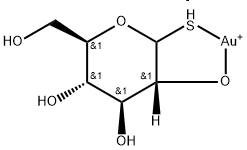
| Identification | Back Directory | [Name]
Aurothioglucose | [CAS]
12192-57-3 | [Synonyms]
gtg
GGT
GGT 1
oronol
brenol
authron
CS-1197
romosol
aurotan
D22S732
aurumine
skf10056
solganal
aureotan
Solganol
glysanolb
auromyose
solganalb
aurothioglucose
goldthioglucose
AUROTHIOGLUCOSE USP
Aurothioglucose 80%
SODIUMAUROTHIOGLUCOSE
(1-d-glucosylthio)gold
Aurothioglucose hydrate
Aurothioglucose (100 mg)
(d-glucopyranosylthio)-gol
(d-glucopyranosylthio)gold
1-aurothio-d-glucopyranose
12192-57-3 Aurothioglucose
(a-D-Glucopyranosylthio)gold
(1-THIO-D-GLUCOPYRANOSATO)GOLD
(1-thio-d-glucopyranosato)-gol
(alpha-D-glucopyranosylthio)gold
Aurio(I)-1-thio-D-glucopyranoside
Aurothioglucose, Gold thioglucose
1-thio-d-glucopyranose,goldcomplex
1-thio-glucopyranose,monogold(1+)salt
Anti-GGT1 antibody produced in rabbit
Gold thioglucose, Solganal, Solganol
1-thio-d-glucopyranose,monogold(1+)salt
1-Deoxy-1-[aurio(I)thio]-D-glucopyranose
Gold, [1-(thio-κS)-D-glucopyranosato-κO2]-
Gold, [1-(thio-.kappa.S)-D-glucopyranosato-.kappa.O2]- | [EINECS(EC#)]
235-365-7 | [Molecular Formula]
C6H11AuO5S | [MDL Number]
MFCD00056080 | [MOL File]
12192-57-3.mol | [Molecular Weight]
392.18 |
| Hazard Information | Back Directory | [Chemical Properties]
yellow crystals, | [Uses]
To produce obesity in experimental animals. | [Brand name]
Solganal (Schering). | [Description]
Aurothioglucose is highly water soluble, and its aqueous solutions decompose on long standing. It therefore is
available as a suspension in sesame oil. Gold content is approximately 50%. Following IM injection, it is highly
protein bound (95%), and peak plasma levels are achieved within 2 to 6 hours. Following a single 50-mg dose, the
biological half-life ranges from 3 to 27 days, but following successive weekly doses, the half-life increases to 14 to
40 days after the third dose. The therapeutic effect does not correlate with serum plasma gold levels but appears to
depend on total accumulated gold. Aurothioglucose is indicated for the adjunctive treatment of adult and juvenile
rheumatoid arthritis. | [History]
In 1927, aurothioglucose was found to relieve joint pain when used to treat bacterial endocarditis. The area of chrysotherapy had begun. Subsequent investigations led to an extensive study of gold compounds in Great Britain by the Empire Rheumatism Council, which reported in 1961 that sodium aurothiomalate was effective in slowing the development of progressive joint diseases. Both aurothioglucose and sodium aurothiomalate are orally ineffective and are administered by IM injection. In 1985, the first orally effective gold compound for arthritis, auranofin, was introduced in the United States. Several other gold compounds have been evaluated clinically but do not appear to offer advantages in terms of efficacy or toxicity. | [Biochem/physiol Actions]
Aurothioglucose, a gold compound used clinically to treat rheumatoid arthritis, has recently been found to be a potent PKCiota-Par6 interaction inhibitor, with an IC50 approximately 1 μM. Disruption of this interaction disrupts a rac1 signaling pathway that is required for transformed growth in non-small-cell lung cancer. | [Clinical Use]
Aurothioglucose is highly water soluble, and its aqueous solutions decompose on long standing. It therefore is available as a suspension in sesame oil. Gold content is approximately 50%. Following IM injection, it is highly protein bound (95%), and peak plasma levels are achieved within 2 to 6 hours. Following a single 50-mg dose, the biological half-life ranges from 3 to 27 days, but following successive weekly doses, the half-life increases to 14 to 40 days after the third dose. The therapeutic effect does not correlate with serum plasma gold levels but appears to depend on total accumulated gold. Aurothioglucose is indicated for the adjunctive treatment of adult and juvenile rheumatoid arthritis. | [Synthesis]
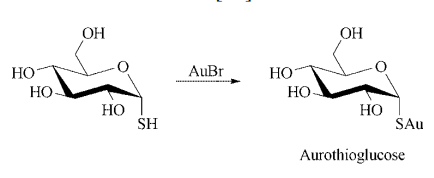
Synthesis: gold thioglucose is prepared by
adding a solution of gold bromide to an aqueous
solution of thioglucose that contains sulfur dioxide. After heating, the product is precipitated by
the addition of ethanol.
 | [Purification Methods]
Purify it by dissolving it in H2O (0.05g in 1mL) and precipitating it by adding EtOH. It yields yellow crystals with a slight mercaptan odour. It decomposes slowly in H2O, and is soluble in propylene glycol but insoluble in EtOH and other common organic solvents. [Caterson & Taylor FEBS Lett 98 351 1979, Cooney et al. Biochem J 259 651 1989.] | [Dosage]
Aurothioglucose is an antirheumatic used to
treat active progressing rheumatoid arthritis and
nondisseminated lupus erythematosus. The drug
is administered at weekly intervals by intramuscular injection (10 mg, 25 mg, then 50 mg) until
800 mg to 1 g has been given. If improvement
takes place, the drug is then administered at levels that balance the urinary excretion of gold.
During this maintenance therapy the interval between injections is lengthened to 3 – 4 weeks. |
| Safety Data | Back Directory | [Hazard Codes ]
Xn | [Risk Statements ]
42/43 | [Safety Statements ]
22-36/37-45 | [RIDADR ]
2811 | [WGK Germany ]
3 | [HazardClass ]
6.1(b) | [PackingGroup ]
III | [HS Code ]
2843300000 | [Safety Profile]
Confirmed carcinogen
with experimental carcinogenic and
neoplastigenic data. A deadly human poison
by an unspecified route. An experimental
poison by intramuscular route. Moderately
toxic by subcutaneous and intravenous
routes. Human systemic effects: nausea or
vomiting, cholestatic jaundlce, and eye
effects. An experimental teratogen. Other
experimental reproductive effects. See also
GOLD COMPOUNDS. When heated to
decomposition it emits very toxic fumes of
SOx. Used to treat rheumatoid arthritis. | [Hazardous Substances Data]
12192-57-3(Hazardous Substances Data) | [Toxicity]
LD50 intravenous in chicken: 1gm/kg |
|
| Company Name: |
Sigma-Aldrich
|
| Tel: |
021-61415566 800-8193336 |
| Website: |
https://www.sigmaaldrich.cn |
| Company Name: |
BOC Sciences
|
| Tel: |
16314854226 |
| Website: |
www.bocsci.com |
|