| Identification | Back Directory | [Name]
Ketone Ester | [CAS]
1208313-97-6 | [Synonyms]
R-BHB
BD-AcAc 2
Ketone Ester
(R)-(R)-3-hydroxybutyl 3-hydroxybutanoate
[(3R)-3-hydroxybutyl] (3R)-3-hydroxybutanoate
(3R)-3-Hydroxybutanoic acid (3R)-3-hydroxybutyl ester
Butanoic acid, 3-hydroxy-, (3R)-3-hydroxybutyl ester, (3R)- | [EINECS(EC#)]
-0 | [Molecular Formula]
C8H16O4 | [MDL Number]
MFCD22417094 | [MOL File]
1208313-97-6.mol | [Molecular Weight]
176.21 |
| Chemical Properties | Back Directory | [Boiling point ]
269℃ | [density ]
1.102 | [Fp ]
101℃ | [storage temp. ]
Store at -20°C | [solubility ]
Methyl Acetate: 50 mg/ml | [form ]
Liquid | [pka]
14.38±0.20(Predicted) | [color ]
Colorless to light yellow | [InChI]
InChI=1S/C8H16O4/c1-6(9)3-4-12-8(11)5-7(2)10/h6-7,9-10H,3-5H2,1-2H3/t6-,7-/m1/s1 | [InChIKey]
AOWPVIWVMWUSBD-RNFRBKRXSA-N | [SMILES]
C(OCC[C@H](O)C)(=O)C[C@H](O)C |
| Hazard Information | Back Directory | [Uses]
Ketone ester(1208313-97-6) is exogenous ketone bodies that may promote ketosis in humans. Animal models demonstrate that ketone ester exert beneficial effects in Alzheimer's disease, effectively reducing amyloid accumulation, neurofibrillary tangles, and tau, while improving learning and memory.
| [Preparation]
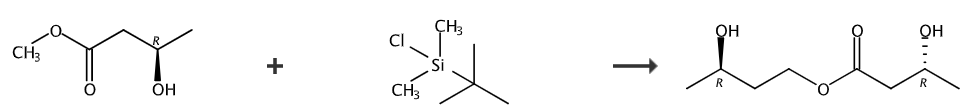
Ketone ester can be prepared by reacting (3R)-3-hydroxybutyl methyl ester with tert-butyl dimethylchlorosilane.
 | [benefits]
Ketone esters can help with muscle recovery after exercising. They increase the rate of energy store replenishment in the body and support the process of rebuilding muscles. They also decrease the amount of muscle breakdown. The various forms of ketone esters that have been utilized include R,S-1,3-butanediol diacetoacetate (BD-AcAc2, ketone diester), D-β-hydroxybutyrate-(R)-1,3 butanediol (ketone monoester), and Bis-hexanoyl (R)-1,3-butanediol (BH-BD), among others.? | [Biological Activity]
Ketone ester–enriched diets that induce sustained ketonemia reduce pathological cardiac remodeling and enhance ventricular function in multiple heart failure models across both mice and rats using both prevention and treatment strategies. | [Safety]
Taking ketone supplements can cause extreme stomach upset in some people. This side effect can limit the number of supplements that a person can take. Taking ketone salts also increases the risk of electrolyte imbalances. Electrolytes are vital for the conduction of electrical signals in the muscles and neurons. | [in vivo]
Blood glucose decreased by about 50% from 5 to 2.8 mM while insulin decreased from 0.54 to 0.26 ng/ml in rats fed a diet where 30% of the calories from starch were replaced with ketone esters (20). Plasma leptin was also decreased from 3.12 to 1.83 ng/ml in rats, where ketone esters were substituted equi-calorically for starch. The ability of the metabolism of ketone bodies to increase insulin sensitivity has been demonstrated previously in the working perfused rat heart[1]. | [References]
[1] Veech R, High electron mobility triazine for lower driving voltage and higher efficiency organic light emitting devices. Journal of Lipid Research, 2014; 55: 2004-2006. |
|
|