| Identification | Back Directory | [Name]
3-(Benzyloxy)-4-oxo-4h-pyran-2-carboxylic acid | [CAS]
119736-16-2 | [Synonyms]
BPCA
Baloxavir Impurity 31
Baloxavir Marboxil Impurity 2
4-oxo-3-phenylmethoxypyran-2-carboxylic acid
3-(Benzyloxy)-4-oxo-4h-pyran-2-carboxylic acid
4-Oxo-3-(benzyloxy)-4H-pyran-2-carboxylic acid
Pinacol 1 - (1-ethoxyethyl) - 4-pyrazole borate
4-Oxo-3-(phenylmethoxy)-4H-pyran-2-carboxylic acid
3-(Benzyloxy)-4-oxo-4H-pyran-2-carboxylic acid, 95+%
4H-Pyran-2-carboxylic acid, 4-oxo-3-(phenylmethoxy)-
4-oxo-3-((phenylmethyl)oxy)-4h-pyran-2-carboxylic acid
3-(Benzyloxy)-4-oxo-4h-pyran-2-carboxylic acid,119736-16-2
3-(benzyloxy)-1-((tert-butoxycarbonyl)amino)-4-oxo-1,4-dihydropyridine-2-carboxylic acid ethyl ester | [EINECS(EC#)]
251-228-4 | [Molecular Formula]
C13H10O5 | [MDL Number]
MFCD22741607 | [MOL File]
119736-16-2.mol | [Molecular Weight]
246.215 |
| Chemical Properties | Back Directory | [Boiling point ]
487.5±45.0 °C(Predicted) | [density ]
1.39±0.1 g/cm3(Predicted) | [storage temp. ]
Sealed in dry,2-8°C | [form ]
Solid | [pka]
2.00±0.20(Predicted) | [color ]
White | [InChI]
InChI=1S/C13H10O5/c14-10-6-7-17-12(13(15)16)11(10)18-8-9-4-2-1-3-5-9/h1-7H,8H2,(H,15,16) | [InChIKey]
KJSJBKBZMGSIPT-UHFFFAOYSA-N | [SMILES]
C1(C(O)=O)OC=CC(=O)C=1OCC1=CC=CC=C1 |
| Hazard Information | Back Directory | [Pharmaceutical Applications]
There are a variety of preclinical, clinical, and marketed new drugs that contain 3-(benzyloxy)-4-oxo-4H-pyran-2-carboxylic acid fragments. As shown in the figure below, several types of drug molecules, as well as the marketed drugs such as dulutevir (GSK1349572) and baloxavir (Baloxavir) contain this important structural fragment.
 | [Uses]
There are a variety of preclinical, clinical, and marketed new drugs that contain 3-(benzyloxy)-4-oxo-4H-pyran-2-carboxylic acid fragments. As shown in the figure below, several types of drug molecules, as well as the marketed drugs such as dulutevir (GSK1349572) and baloxavir (Baloxavir) contain this important structural fragment. | [Synthesis]
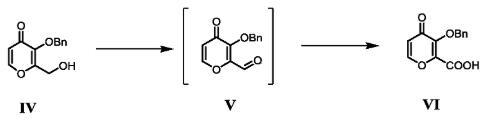
Add 2-(hydroxymethyl)-3-(benzyloxy)-4H-pyran-4-one (Compound IV) 39.8Kg, dichloromethane (340L) and water (170L) to the reaction kettle to start stirring. Then add sodium bicarbonate (77Kg), sodium bromide (1.6Kg) and 2,2,6,6-tetramethylpiperidine-nitrogen-oxide (TEMPO) (2.3Kg), cool to 5℃, drop Add 10% sodium hypochlorite (340Kg). After the dropwise addition, stir at 5°C for 1 hour. The reaction is completed. Add 30% aqueous sodium thiosulfate solution (242L) and stir for 1 h. Add concentrated hydrochloric acid to adjust pH=4, separate the organic phase, extract the aqueous phase twice with dichloromethane (120L), combine the organic phases, and concentrate under reduced pressure to obtain the crude product. The crude product was recrystallized from acetonitrile to obtain 3-(benzyloxy)-4-oxo-4H-pyran-2-carboxylic acid (Compound VI) 32.4Kg, purity 99%, yield 77%. LC-MS: [M+H] + = 247.06, [M] + = 245.05; 1 H NMR (DMSO-d 6 ) δ8.00 (d, J = 5.6 Hz, 1H), 7.17 (s, 5H), 6.34 (d, J = 5.7 Hz, 1H), 4.91 (s, 2H), 2.30 (s, 1H). |
|
|